Linezolid
These highlights do not include all the information needed to use LINEZOLID TABLETS safely and effectively. See full prescribing information for LINEZOLID TABLETS. LINEZOLID tablets, for oral use Initial U.S. Approval: 2000
7402aa01-50de-46c8-a341-d30da5b32b1c
HUMAN PRESCRIPTION DRUG LABEL
Dec 8, 2023
Ascend Laboratories, LLC
DUNS: 141250469
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Linezolid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
The safety of Linezolid formulations was evaluated in 2,046 adult patients
enrolled in seven Phase 3 comparator-controlled clinical trials, who were
treated for up to 28 days.
Of the patients treated for uncomplicated skin and skin structure infections
(uSSSIs), 25.4% of Linezolid-treated and 19.6% of comparator-treated patients
experienced at least one drug-related adverse event. For all other
indications, 20.4% of Linezolid-treated and 14.3% of comparator-treated
patients experienced at least one drug-related adverse event.
Table 2 shows the incidence of all-causality, treatment-emergent adverse
reactions reported in at least 1% of adult patients in these trials by dose of
Linezolid.
**Table 2. Incidence (%) of Treatment–Emergent Adverse Reactions Occurring in
1% of Adult Patients Treated with Linezolid in Comparator-Controlled Clinical Trials**
|
ADVERSE REACTIONS |
Uncomplicated Skin and Skin Structure Infections |
All Other Indications | ||
|
Linezolid |
Clarithromycin |
Linezolid |
All Other Comparators* | |
|
Headache |
8.8 |
8.4 |
5.7 |
4.4 |
|
Diarrhea |
8.2 |
6.1 |
8.3 |
6.4 |
|
Nausea |
5.1 |
4.5 |
6.6 |
4.6 |
|
Vomiting |
2.0 |
1.5 |
4.3 |
2.3 |
|
Dizziness |
2.6 |
3.0 |
1.8 |
1.5 |
|
Rash |
1.1 |
1.1 |
2.3 |
2.6 |
|
Anemia |
0.4 |
0 |
2.1 |
1.4 |
|
Taste alteration |
1.8 |
2.0 |
1.0 |
0.3 |
|
Vaginal moniliasis |
1.8 |
1.3 |
1.1 |
0.5 |
|
Oral moniliasis |
0.5 |
0 |
1.7 |
1.0 |
|
Abnormal liver function tests |
0.4 |
0.2 |
1.6 |
0.8 |
|
Fungal infection |
1.5 |
0.2 |
0.3 |
0.2 |
|
Tongue discoloration |
1.3 |
0 |
0.3 |
0 |
|
Localized abdominal pain |
1.3 |
0.6 |
1.2 |
0.8 |
|
Generalized abdominal pain |
0.9 |
0.4 |
1.2 |
1.0 |
- Comparators included cefpodoxime proxetil 200 mg by mouth every 12 hours; ceftriaxone 1 g intravenously every 12 hours; dicloxacillin 500 mg by mouth every 6 hours; oxacillin 2 g intravenously every 6 hours; vancomycin 1 g intravenously every 12 hours.
Of the patients treated for uSSSIs, 3.5% of Linezolid-treated and 2.4% of comparator-treated patients discontinued treatment due to drug-related adverse events. For all other indications, discontinuations due to drug-related adverse events occurred in 2.1% of Linezolid-treated and 1.7% of comparator- treated patients. The most common reported drug-related adverse events leading to discontinuation of treatment were nausea, headache, diarrhea, and vomiting.
Pediatric Patients
The safety of Linezolid formulations was evaluated in 215 pediatric patients ranging in age from birth through 11 years, and in 248 pediatric patients aged 5 through 17 years (146 of these 248 were age 5 through 11 and 102 were age 12 to 17). These patients were enrolled in two Phase 3 comparator-controlled clinical trials and were treated for up to 28 days. In the study of hospitalized pediatric patients (birth through 11 years) with Gram-positive infections, who were randomized 2 to 1 (linezolid: vancomycin), mortality was 6.0% (13/215) in the linezolid arm and 3.0% (3/101) in the vancomycin arm. However, given the severe underlying illness in the patient population, no causality could be established.
Of the pediatric patients treated for uSSSIs, 19.2% of Linezolid-treated and 14.1% of comparator-treated patients experienced at least one drug-related adverse event. For all other indications, 18.8% of Linezolid-treated and 34.3% of comparator-treated patients experienced at least one drug-related adverse event.
Table 3 shows the incidence of all-causality, treatment-emergent adverse reactions reported in more than 1% of pediatric patients (and more than 1 patient) in either treatment group in the comparator-controlled Phase 3 trials.
**Table 3. Incidence (%) of Treatment-Emergent Adverse Reactions Occurring in
1% of Pediatric Patients (and >1 Patient) in Either Treatment Group in Comparator-Controlled Clinical Trials**
|
ADVERSE REACTIONS |
Uncomplicated Skin and Skin Structure Infections***** |
All Other Indications**†** | ||
|
Linezolid |
Cefadroxil (n=251) |
** Linezolid** |
Vancomycin (n=101) | |
|
Diarrhea |
7.8 |
8.0 |
10.8 |
12.1 |
|
Vomiting |
2.9 |
6.4 |
9.4 |
9.1 |
|
Headache |
6.5 |
4.0 |
0.9 |
0 |
|
Anemia |
0 |
0 |
5.6 |
7.1 |
|
Thrombocytopenia |
0 |
0 |
4.7 |
2.0 |
|
Nausea |
3.7 |
3.2 |
1.9 |
0 |
|
Generalized abdominal pain |
2.4 |
2.8 |
0.9 |
2.0 |
|
Localized abdominal pain |
2.4 |
2.8 |
0.5 |
1.0 |
|
Loose stools |
1.6 |
0.8 |
2.3 |
3.0 |
|
Eosinophilia |
0.4 |
0.8 |
1.9 |
1.0 |
|
Pruritus at non-application site |
0.8 |
0.4 |
1.4 |
2.0 |
|
Vertigo |
1.2 |
0.4 |
0 |
0 |
- Patients 5 through 11 years of age received Linezolid 10 mg/kg by mouth every 12 hours or cefadroxil 15 mg/kg by mouth every 12 hours. Patients 12 years or older received Linezolid 600 mg by mouth every 12 hours or cefadroxil 500 mg by mouth every 12 hours.
† Patients from birth through 11 years of age received Linezolid 10 mg/kg intravenously by mouth every 8 hours or vancomycin 10 to 15 mg/kg intravenously every 6 to 24 hours, depending on age and renal clearance.
Of the pediatric patients treated for uSSSIs, 1.6% of Linezolid-treated and 2.4% of comparator-treated patients discontinued treatment due to drug-related adverse events. For all other indications, discontinuations due to drug- related adverse events occurred in 0.9% of Linezolid-treated and 6.1% of comparator-treated patients.
Laboratory Abnormalities
Linezolid has been associated with thrombocytopenia when used in doses up to and including 600 mg every 12 hours for up to 28 days. In Phase 3 comparator- controlled trials, the percentage of adult patients who developed a substantially low platelet count (defined as less than 75% of lower limit of normal and/or baseline) was 2.4% (range among studies: 0.3 to 10.0%) with Linezolid and 1.5% (range among studies: 0.4 to 7.0%) with a comparator. In a study of hospitalized pediatric patients ranging in age from birth through 11 years, the percentage of patients who developed a substantially low platelet count (defined as less than 75% of lower limit of normal and/or baseline) was 12.9% with Linezolid and 13.4% with vancomycin. In an outpatient study of pediatric patients aged from 5 through 17 years, the percentage of patients who developed a substantially low platelet count was 0% with Linezolid and 0.4% with cefadroxil. Thrombocytopenia associated with the use of Linezolid appears to be dependent on duration of therapy (generally greater than 2 weeks of treatment). The platelet counts for most patients returned to the normal range/baseline during the follow-up period. No related clinical adverse events were identified in Phase 3 clinical trials in patients developing thrombocytopenia. Bleeding events were identified in thrombocytopenic patients in a compassionate use program for Linezolid; the role of linezolid in these events cannot be determined [see Warnings and Precautions (5.1)].
Changes seen in other laboratory parameters, without regard to drug relationship, revealed no substantial differences between Linezolid and the comparators. These changes were generally not clinically significant, did not lead to discontinuation of therapy, and were reversible. The incidence of adult and pediatric patients with at least one substantially abnormal hematologic or serum chemistry value is presented in Tables 4, 5, 6, and 7.
Table 4. Percent of Adult Patients who Experienced at Least One Substantially Abnormal*******Hematology Laboratory Value in Comparator- Controlled Clinical Trials with Linezolid**
|
Laboratory Assay |
Uncomplicated Skin and Skin Structure Infections |
All Other Indications | ||
|
Linezolid |
Clarithromycin |
Linezolid |
All Other Comparators**†** | |
|
Hemoglobin (g/dL) |
0.9 |
0.0 |
7.1 |
6.6 |
|
Platelet count (x 103/mm3) |
0.7 |
0.8 |
3.0 |
1.8 |
|
WBC (x 103/mm3) |
0.2 |
0.6 |
2.2 |
1.3 |
|
Neutrophils (x 103/mm3) |
0.0 |
0.2 |
1.1 |
1.2 |
***<**75% (<50% for neutrophils) of Lower Limit of Normal (LLN) for values normal at baseline; <75% (<50% for neutrophils) of LLN and of baseline for values abnormal at baseline.
† Comparators included cefpodoxime proxetil 200 mg by mouth every 12 hours; ceftriaxone 1 g intravenously every 12 hours; dicloxacillin 500 mg by mouth every 6 hours; oxacillin 2 g intravenously every 6 hours; vancomycin 1 g intravenously every 12 hours.
Table 5. Percent of Adult Patients who Experienced at Least One Substantially Abnormal*******Serum Chemistry Laboratory Value in Comparator-Controlled Clinical Trials with Linezolid**
|
Laboratory Assay |
Uncomplicated Skin and Skin Structure Infections |
All Other Indications | ||
|
Linezolid |
Clarithromycin |
Linezolid |
All Other Comparators**†** | |
|
AST (U/L) |
1.7 |
1.3 |
5.0 |
6.8 |
|
ALT (U/L) |
1.7 |
1.7 |
9.6 |
9.3 |
|
LDH (U/L) |
0.2 |
0.2 |
1.8 |
1.5 |
|
Alkaline phosphatase (U/L) |
0.2 |
0.2 |
3.5 |
3.1 |
|
Lipase (U/L) |
2.8 |
2.6 |
4.3 |
4.2 |
|
Amylase (U/L) |
0.2 |
0.2 |
2.4 |
2.0 |
|
Total bilirubin (mg/dL) |
0.2 |
0.0 |
0.9 |
1.1 |
|
BUN (mg/dL) |
0.2 |
0.0 |
2.1 |
1.5 |
|
Creatinine (mg/dL) |
0.2 |
0.0 |
0.2 |
0.6 |
***>**2 x Upper Limit of Normal (ULN) for values normal at baseline;
**>**2 x ULN and >2 x baseline for values abnormal at baseline.
† Comparators included cefpodoxime proxetil 200 mg by mouth every 12 hours; ceftriaxone 1 g intravenously every 12 hours; dicloxacillin 500 mg by mouth every 6 hours; oxacillin 2 g intravenously every 6 hours; vancomycin 1 g intravenously every 12 hours.
Table 6. Percent of Pediatric Patients who Experienced at Least One Substantially Abnormal*******Hematology Laboratory Value in Comparator- Controlled Clinical Trials with Linezolid**
|
Laboratory Assay |
Uncomplicated Skin and Skin Structure Infections**†** |
All Other Indications**‡** | ||
|
Linezolid |
** Cefadroxil** |
Linezolid |
** Vancomycin** | |
|
Hemoglobin (g/dL) |
0.0 |
0.0 |
15.7 |
12.4 |
|
Platelet count (x 103/mm3) |
0.0 |
0.4 |
12.9 |
13.4 |
|
WBC (x 103/mm3) |
0.8 |
0.8 |
12.4 |
10.3 |
|
Neutrophils (x 103/mm3) |
1.2 |
0.8 |
5.9 |
4.3 |
***<75% (<50% for neutrophils) of Lower Limit of Normal (LLN) for values normal at baseline;<**75% (<50% for neutrophils) of LLN and <75% (<50% for neutrophils, <90% for hemoglobin if baseline <LLN) of baseline for values abnormal at baseline.
† Patients 5 through 11 years of age received Linezolid 10 mg/kg by mouth every 12 hours or cefadroxil 15 mg/kg by mouth every 12 hours. Patients 12 years or older received Linezolid 600 mg by mouth every 12 hours or cefadroxil 500 mg by mouth every 12 hours.
‡ Patients from birth through 11 years of age received Linezolid 10 mg/kg intravenously by mouth every 8 hours or vancomycin 10 to 15 mg/kg intravenously every 6 to 24 hours, depending on age and renal clearance.
Table 7. Percent of Pediatric Patients who Experienced at Least One Substantially Abnormal*******Serum Chemistry Laboratory Value in Comparator-Controlled Clinical Trials with Linezolid**
|
Laboratory Assay |
Uncomplicated Skin and Skin Structure Infections**†** |
All Other Indications**‡** | ||
|
Linezolid |
Cefadroxil |
Linezolid |
** Vancomycin** | |
|
ALT (U/L) |
0.0 |
0.0 |
10.1 |
12.5 |
|
Lipase (U/L) |
0.4 |
1.2 |
-- |
-- |
|
Amylase (U/L) |
-- |
-- |
0.6 |
1.3 |
|
Total bilirubin (mg/dL) |
-- |
-- |
6.3 |
5.2 |
|
Creatinine (mg/dL) |
0.4 |
0.0 |
2.4 |
1.0 |
-
2 x Upper Limit of Normal (ULN) for values normal at baseline; >2 x ULN and >2 (>1.5 for total bilirubin) x baseline for values abnormal at baseline.
† Patients 5 through 11 years of age received Linezolid 10 mg/kg by mouth every 12 hours or cefadroxil 15 mg/kg by mouth every 12 hours. Patients 12 years or older received Linezolid 600 mg mouth every 12 hours or cefadroxil 500 mg by mouth every 12 hours.
‡ Patients from birth through 11 years of age received Linezolid 10 mg/kg intravenously/by mouth every 8 hours or vancomycin 10 to 15 mg/kg intravenously every 6 to 24 hours, depending on age and renal clearance.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Linezolid. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
• Myelosuppression (including anemia, leukopenia, pancytopenia, and thrombocytopenia).Thrombocytopenia has been reported more often in patients with severe renal impairment and in patients with moderate to severe hepatic impairment [see Warnings and Precautions (5.1)]; sideroblastic anemia.
• Peripheral neuropathy, and optic neuropathy sometimes progressing to loss of vision [see Warnings and Precautions (5.2)].
• Lactic acidosis [see Warnings and Precautions (5.7)]. Although these reports have primarily been in patients treated for longer than the maximum recommended duration of 28 days, these events have also been reported in patients receiving shorter courses of therapy.
• Serotonin syndrome has been reported in patients receiving concomitant serotonergic agents, including antidepressants such as selective serotonin reuptake inhibitors (SSRIs) and opioids, and Linezolid [see Warnings and Precautions (5.3)].
• Convulsions [see Warnings and Precautions (5.8)].
• Anaphylaxis, angioedema, bullous skin disorders including severe cutaneous adverse reactions (SCAR) such as toxic epidermal necrolysis and Stevens- Johnson syndrome, and hypersensitivity vasculitis.
• Superficial tooth discoloration and tongue discoloration have been reported with the use of linezolid. The tooth discoloration was removable with professional dental cleaning (manual descaling) in cases with known outcome.
• Hypoglycemia, including symptomatic episodes [see Warnings and Precautions (5.9)].
• Hyponatremia and/or Syndrome of Inappropriate Antidiuretic Hormone Secretion(SIADH) [see Warnings and Precautions (5.10)].
Most common adverse reactions (>5% of adult and/or pediatric patients treated with Linezolid) include: diarrhea, vomiting, headache, nausea, and anemia. (6) To report SUSPECTED ADVERSE REACTIONS, contact Ascend Laboratories, LLC at 1-877-272-7901 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Important Administration Instructions
Advise patients that linezolid may be taken with or without food.
Peripheral and Optic Neuropathy
Advise patients to inform their physician if they experience changes in vision while taking linezolid [see Warnings and Precautions (5.2)].
Serotonin Syndrome
Advise patients to inform their physician if taking serotonergic agents, including serotonin re-uptake inhibitors or other antidepressants and opioids [ see Warnings and Precautions (5.3)].
Potential Interactions Producing Elevation of Blood Pressure
- Advise patients to inform their physician if they have a history of hypertension.
- Advise patients to avoid large quantities of foods or beverages with high tyramine content while taking linezolid. Foods high in tyramine content include those that may have undergone protein changes by aging, fermentation, pickling, or smoking to improve flavor, such as aged cheeses, fermented or air-dried meats, sauerkraut, soy sauce, tap beers, and red wines. The tyramine content of any protein-rich food may be increased if stored for long periods or improperly refrigerated.
- Advise patients to inform their physician if taking medications containing pseudoephedrine HCl or phenylpropanolamine HCl, such as cold remedies and decongestants [ see Warnings and Precautions (5.6)].
Lactic Acidosis
Advise patients to inform their physician if they experience repeated episodes of nausea or vomiting while receiving linezolid [see Warnings and Precautions (5.7)].
Convulsions
Advise patients to inform their physician if they have a history of seizures or convulsions [see Warnings and Precautions (5.8)].
Hypoglycemia
Advise patients to inform their physician if they have diabetes mellitus. Hypoglycemic reactions, such as diaphoresis and tremulousness, along with low blood glucose measurements may occur when treated with linezolid. If such reactions occur, patients should contact a physician or other health professional for proper treatment [see Warnings and Precautions (5.9)].
Hyponatremia and/or SIADH
Advise patients at risk for hyponatremia to inform their physician if they
experience signs and symptoms of hyponatremia and/or SIADH, including
confusion, somnolence, generalized weakness, and respiratory distress [see Warnings and Precautions (5.10)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs including linezolid should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When linezolid is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by linezolid or other antibacterial drugs in the future.
Diarrhea
Diarrhea is a common problem caused by antibacterial drugs, which usually ends when the antibacterial drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible [see Warnings and Precautions (5.5)].
Infertility
Advise male patients that linezolid may reversibly impair fertility [see Use in Specific Populations (8.3)].
** Manufactured by:**
****Alkem Laboratories Ltd.,
INDIA.
Distributed by:
Ascend Laboratories, LLC
Parsippany, NJ 07054
Revised: October, 2023
** PT 1985-11**
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Linezolid is an antibacterial drug [see Microbiology ( 12.4)].
12.2 Pharmacodynamics
In a randomized, positive-and placebo-controlled crossover thorough QT study, 40 healthy subjects were administered a single Linezolid 600 mg dose via a 1 hour IV infusion, a single Linezolid 1,200 mg dose via a 1 hour IV infusion, placebo, and a single oral dose of positive control. At both the 600 mg and 1,200 mg Linezolid doses, no significant effect on QTc interval was detected at peak plasma concentration or at any other time.
12.3 Pharmacokinetics
The mean pharmacokinetic parameters of linezolid in adults after single and
multiple oral and intravenous doses are summarized in Table 8. Plasma
concentrations of linezolid at steady-state after oral doses of 600 mg given
every 12 hours are shown in Figure 1.
Table 8. Mean (Standard Deviation) Pharmacokinetic Parameters of Linezolid
in Adults
|
Dose of Linezolid |
C****max |
C****min |
T****max |
AUC* |
t****1/2 |
CL |
|
400 mg tablet | ||||||
|
single dose† |
8.10 |
--- |
1.52 |
55.10 |
5.20 |
146 |
|
every 12 hours |
11.00 |
3.08 |
1.12 |
73.40 |
4.69 |
110 |
|
600 mg tablet | ||||||
|
single dose |
12.70 |
--- |
1.28 |
91.40 |
4.26 |
127 |
|
every 12 hours |
21.20 |
6.15 |
1.03 |
138.00 |
5.40 |
80 |
|
600 mg IV injection**‡** | ||||||
|
single dose |
12.90 |
--- |
0.50 |
80.20 |
4.40 |
138 |
|
every 12 hours |
15.10 |
3.68 |
0.51 |
89.70 |
4.80 |
123 |
|
** 600 mg oral suspension** | ||||||
|
single dose |
11.00 |
--- |
0.97 |
80.80 |
4.60 |
141 |
*****AUC for single dose = AUC0–∞; for multiple dose = AUC0–t
**†**Data dose-normalized from 375 mg
**‡**Data dose-normalized from 625 mg, intravenous dose was given as 0.5-hour infusion.
Cmax = Maximum plasma concentration; Cmin = Minimum plasma concentration; Tmax = Time to Cmax; AUC = Area under concentration-time curve; t1/2 = Elimination half-life; CL = Systemic clearance
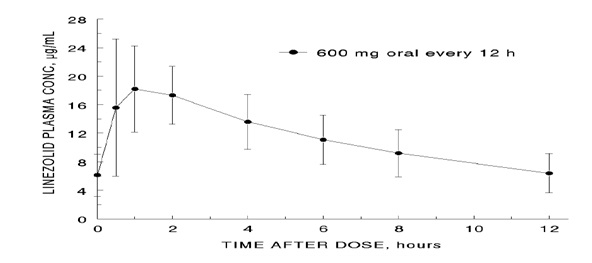
Figure 1. Plasma Concentrations of Linezolid in Adults at Steady-State Following Oral Dosing Every 12 Hours (Mean±Standard Deviation, n=16)
****Absorption
Linezolid is extensively absorbed after oral dosing. Maximum plasma concentrations are reached approximately 1 to 2 hours after dosing, and the absolute bioavailability is approximately 100%. Therefore, linezolid may be given orally or intravenously without dose adjustment.
Linezolid may be administered without regard to the timing of meals. The time to reach the maximum concentration is delayed from 1.5 hours to 2.2 hours and Cmax is decreased by about 17% when high fat food is given with linezolid. However, the total exposure measured as AUC0-∞ is similar under both conditions.
Distribution
Animal and human pharmacokinetic studies have demonstrated that linezolid
readily distributes to well-perfused tissues. The plasma protein binding of
linezolid is approximately 31% and is concentration-independent. The volume of
distribution of linezolid at steady-state averaged 40 to 50 liters in healthy
adult volunteers.
Linezolid concentrations have been determined in various fluids from a limited
number of subjects in Phase 1 volunteer studies following multiple dosing of
linezolid. The ratio of linezolid in saliva relative to plasma was 1.2 to 1
and the ratio of linezolid in sweat relative to plasma was 0.55 to 1.
Metabolism
Linezolid is primarily metabolized by oxidation of the morpholine ring, which results in two inactive ring-opened carboxylic acid metabolites: the aminoethoxyacetic acid metabolite (A), and the hydroxyethyl glycine metabolite (B). Formation of metabolite A is presumed to be formed via an enzymatic pathway whereas metabolite B is mediated by a non-enzymatic chemical oxidation mechanism in vitro. In vitro studies have demonstrated that linezolid is minimally metabolized and may be mediated by human cytochrome P450. However, the metabolic pathway of linezolid is not fully understood.
Excretion
Nonrenal clearance accounts for approximately 65% of the total clearance of linezolid. Under steady-state conditions, approximately 30% of the dose appears in the urine as linezolid, 40% as metabolite B, and 10% as metabolite A. The mean renal clearance of linezolid is 40 mL/min which suggests net tubular reabsorption. Virtually no linezolid appears in the feces, while approximately 6% of the dose appears in the feces as metabolite B, and 3% as metabolite A.
A small degree of nonlinearity in clearance was observed with increasing doses of linezolid, which appears to be due to lower renal and nonrenal clearance of linezolid at higher concentrations. However, the difference in clearance was small and was not reflected in the apparent elimination half-life.
Specific Populations
Geriatric Patients
The pharmacokinetics of linezolid are not significantly altered in elderly patients (65 years or older). Therefore, dose adjustment for geriatric patients is not necessary.
Pediatric Patients
The pharmacokinetics of linezolid following a single intravenous dose were investigated in pediatric patients ranging in age from birth through 17 years (including premature and full-term neonates), in healthy adolescent subjects ranging in age from 12 through 17 years, and in pediatric patients ranging in age from 1 week through 12 years. The pharmacokinetic parameters of linezolid are summarized in Table 9 for the pediatric populations studied and healthy adult subjects after administration of single intravenous doses.
The Cmax and the volume of distribution (Vss) of linezolid are similar regardless of age in pediatric patients. However, plasma clearance of linezolid varies as a function of age. With the exclusion of pre-term neonates less than one week of age, weight-based clearance is most rapid in the youngest age groups ranging from <1 week old to 11 years, resulting in lower single-dose systemic exposure (AUC) and a shorter half-life as compared with adults. As the age of pediatric patients increases, the weight-based clearance of linezolid gradually decreases, and by adolescence mean clearance values approach those observed for the adult population. There is increased inter- subject variability in linezolid clearance and systemic drug exposure (AUC) across all pediatric age groups as compared with adults.
Similar mean daily AUC values were observed in pediatric patients from birth to 11 years of age dosed every 8 hours relative to adolescents or adults dosed every 12 hours. Therefore, the dosage for pediatric patients up to 11 years of age should be 10 mg/kg every 8 hours. Pediatric patients 12 years and older should receive 600 mg every 12 hours [see Dosage and Administration (2)].
Table 9. Pharmacokinetic Parameters of Linezolid in Pediatrics and Adults Following a Single Intravenous Infusion of 10 mg/kg or 600 mg Linezolid (Mean: (%CV); [Min, Max Values])
|
Age Group |
C****max |
V****ss |
AUC* |
t****1/2 |
CL |
|
Neonatal Patients | |||||
|
Pre-term** |
12.7 (30%) |
0.81 (24%) |
108 (47%) |
5.6 (46%) |
2.0 (52%) |
|
<1 week (N=9)† |
[9.6, 22.2] |
[0.43, 1.05] |
[41, 191] |
[2.4, 9.8] |
[0.9, 4.0] |
|
Full-term*** |
11.5 (24%) |
0.78 (20%) |
55 (47%) |
3.0 (55%) |
3.8 (55%) |
|
<1 week (N=10)† |
[8.0, 18.3] |
[0.45, 0.96] |
[19, 103] |
[1.3, 6.1] |
[1.5, 8.8] |
|
Full-term*** | |||||
|
≥1 week to ≤28 days |
12.9 (28%) |
0.66 (29%) |
34 (21%) |
1.5 (17%) |
5.1 (22%) |
|
Infant Patients | |||||
|
11.0 (27%) |
0.79 (26%) |
33 (26%) |
1.8 (28%) |
5.4 (32%) |
|
Pediatric Patients | |||||
|
3 months through 11 years† (N=59) |
15.1 (30%) |
0.69 (28%) |
58 (54%) |
2.9 (53%) |
3.8 (53%) |
|
Adolescent Subjects and Patients | |||||
|
12 through 17 years‡ (N=36) |
16.7 (24%) |
0.61 (15%) |
95 (44%) |
4.1 (46%) |
2.1 (53%) |
|
Adult Subjects§ |
12.5 (21%) |
0.65 (16%) |
91 (33%) |
4.9 (35%) |
1.7 (34%) |
|
(N=29) |
[8.2, 19.3] |
[0.45, 0.84] |
[53, 155] |
[1.8, 8.3] |
[0.9, 3.3] |
*AUC = Single dose AUC0–∞
**In this data set, “pre-term” is defined as <34 weeks gestational age (Note: Only 1 patient enrolled was pre-term with a postnatal age between 1 week and 28 days)
*** In this data set, “full-term” is defined as ≥34 weeks gestational age
† Dose of 10 mg/kg
‡ Dose of 600 mg or 10 mg/kg up to a maximum of 600 mg
**§**Dose normalized to 600 mg
Cmax = Maximum plasma concentration; Vss= Volume of distribution; AUC = Area under concentration-time curve; t1/2 = Apparent elimination half-life; CL = Systemic clearance normalized for body weight
Gender
Females have a slightly lower volume of distribution of linezolid than males. Plasma concentrations are higher in females than in males, which is partly due to body weight differences. After a 600-mg dose, mean oral clearance is approximately 38% lower in females than in males. However, there are no significant gender differences in mean apparent elimination-rate constant or half-life. Thus, drug exposure in females is not expected to substantially increase beyond levels known to be well tolerated. Therefore, dose adjustment by gender does not appear to be necessary.
Renal Impairment
The pharmacokinetics of the parent drug, linezolid, are not altered in patients with any degree of renal impairment; however, the two primary metabolites of linezolid accumulate in patients with renal impairment, with the amount of accumulation increasing with the severity of renal dysfunction (see Table 10). The pharmacokinetics of linezolid and its two metabolites have also been studied in patients with end-stage renal disease (ESRD) receiving hemodialysis. In the ESRD study, 14 patients were dosed with linezolid 600 mg every 12 hours for 14.5 days (see Table 11). Because similar plasma concentrations of linezolid are achieved regardless of renal function, no dose adjustment is recommended for patients with renal impairment. However, given the absence of information on the clinical significance of accumulation of the primary metabolites, use of linezolid in patients with renal impairment should be weighed against the potential risks of accumulation of these metabolites. Both linezolid and the two metabolites are eliminated by hemodialysis. No information is available on the effect of peritoneal dialysis on the pharmacokinetics of linezolid. Approximately 30% of a dose was eliminated in a 3-hour hemodialysis session beginning 3 hours after the dose of linezolid was administered; therefore, linezolid should be given after hemodialysis.
** Table 10. Mean (Standard Deviation) AUCs and Elimination Half-lives of Linezolid and Metabolites A and B in Patients with Varying Degrees of Renal Impairment After a Single 600 mg Oral Dose of Linezolid**
|
Parameter |
Healthy Subjects CLCR> 80 mL/min |
Moderate Renal Impairment 30 < CLCR< 80 mL/min |
Severe Renal Impairment 10 < CLCR< 30 mL/min | |
|
LINEZOLID | ||||
|
AUC0-∞, mcg h/mL |
110 (22) |
128 (53) |
127 (66) | |
|
t1/2, hours |
6.4 (2.2) |
6.1 (1.7) |
7.1 (3.7) | |
|
METABOLITE A | ||||
|
AUC0-48, mcg h/mL |
7.6 (1.9) |
11.7 (4.3) |
56.5 (30.6) | |
|
t1/2, hours |
6.3 (2.1) |
6.6 (2.3) |
9.0 (4.6) | |
|
METABOLITE B****1 | ||||
|
AUC0-48, mcg h/mL |
30.5 (6.2) |
51.1 (38.5) |
203 (92) | |
|
t1/2, hours |
6.6 (2.7) |
9.9 (7.4) |
11.0 (3.9) |
1 Metabolite B is the major metabolite of linezolid.
Table 11. Mean (Standard Deviation) AUCs and Elimination Half-lives of Linezolid and Metabolites A and B in Subjects with End-Stage Renal Disease (ESRD) After the Administration of 600 mg Linezolid Every 12 Hours for 14.5 Days
|
Parameter |
ESRD Subjects****1 |
|
LINEZOLID | |
|
AUC0-12, mcg h/mL (after last dose) |
181 (52.3) |
|
t1/2, h (after last dose) |
8.3 (2.4) |
|
METABOLITE A | |
|
AUC0-12, mcg h/mL (after last dose) |
153 (40.6) |
|
t1/2, h (after last dose) |
15.9 (8.5) |
|
METABOLITE B****2 | |
|
AUC0-12, mcg h/mL (after last dose) |
356 (99.7) |
|
t1/2, h (after last dose) |
34.8 (23.1) |
1 between hemodialysis sessions
2 Metabolite B is the major metabolite of linezolid.
Hepatic Impairment
The pharmacokinetics of linezolid are not altered in patients (n=7) with mild- to-moderate hepatic impairment (Child-Pugh class A or B). On the basis of the available information, no dose adjustment is recommended for patients with mild-to-moderate hepatic impairment. The pharmacokinetics of linezolid in patients with severe hepatic impairment have not been evaluated.
Drug Interactions
Drugs Metabolized by Cytochrome P450
Linezolid is not an inducer of cytochrome P450 (CYP450) in rats. In addition, linezolid does not inhibit the activities of clinically significant human CYP isoforms (e.g., 1A2, 2C9, 2C19, 2D6, 2E1, 3A4). Therefore, linezolid is not expected to affect the pharmacokinetics of other drugs metabolized by these major enzymes. Concurrent administration of linezolid does not substantially alter the pharmacokinetic characteristics of (S)-warfarin, which is extensively metabolized by CYP2C9. Drugs such as warfarin and phenytoin, which are CYP2C9 substrates, may be given with linezolid without changes in dosage regimen.
Antibacterial Drugs
Aztreonam: The pharmacokinetics of linezolid or aztreonam are not altered when administered together.
Gentamicin: The pharmacokinetics of linezolid or gentamicin are not altered when administered together.
Antioxidants
The potential for drug-drug interactions with linezolid and the antioxidants Vitamin C and Vitamin E was studied in healthy volunteers. Subjects were administered a 600 mg oral dose of linezolid on Day 1, and another 600 mg dose of linezolid on Day 8. On Days 2 to 9, subjects were given either Vitamin C (1,000 mg/day) or Vitamin E (800 IU/ day). The AUC0-∞ of linezolid increased 2.3% when co-administered with Vitamin C and 10.9% when co-administered with Vitamin E. No linezolid dose adjustment is recommended during co- administration with Vitamin C or Vitamin E.
Strong CYP 3A4 Inducers
Rifampin: The effect of rifampin on the pharmacokinetics of linezolid was evaluated in a study of 16 healthy adult males. Volunteers were administered oral linezolid 600 mg twice daily for 5 doses with and without rifampin 600 mg once daily for 8 days. Co-administration of rifampin with linezolid resulted in a 21% decrease in linezolid Cmax [90% CI, 15% to 27%] and a 32% decrease in linezolid AUC0-12 [90% CI, 27% to 37%]. The clinical significance of this interaction is unknown. The mechanism of this interaction is not fully understood and may be related to the induction of hepatic enzymes. Other strong inducers of hepatic enzymes (e.g. carbamazepine, phenytoin, phenobarbital) could cause a similar or smaller decrease in linezolid exposure.
Monoamine Oxidase Inhibition
Linezolid is a reversible, nonselective inhibitor of monoamine oxidase. Therefore, linezolid has the potential for interaction with adrenergic and serotonergic agents.
Adrenergic Agents
Some individuals receiving Linezolid may experience a reversible enhancement of the pressor response to indirect-acting sympathomimetic agents, vasopressor or dopaminergic agents. Commonly used drugs such as phenylpropanolamine and pseudoephedrine have been specifically studied. Initial doses of adrenergic agents, such as dopamine or epinephrine, should be reduced and titrated to achieve the desired response.
Tyramine: A significant pressor response has been observed in normal adult subjects receiving linezolid and tyramine doses of more than 100 mg. Therefore, patients receiving linezolid need to avoid consuming large amounts of foods or beverages with high tyramine content [see Patient Counseling Information (17)].
Pseudoephedrine HCl or phenylpropanolamine HCl: A reversible enhancement of the pressor response of either pseudoephedrine HCl (PSE) or phenylpropanolamine HCl (PPA) is observed when linezolid is administered to healthy normotensive subjects [see Warnings and Precautions (5.6) and Drug Interactions (7)]. A similar study has not been conducted in hypertensive patients. The interaction studies conducted in normotensive subjects evaluated the blood pressure and heart rate effects of placebo, PPA or PSE alone, linezolid alone, and the combination of steady-state linezolid (600 mg every 12 hours for 3 days) with two doses of PPA (25 mg) or PSE (60 mg) given 4 hours apart. Heart rate was not affected by any of the treatments. Blood pressure was increased with both combination treatments. Maximum blood pressure levels were seen 2 to 3 hours after the second dose of PPA or PSE, and returned to baseline 2 to 3 hours after peak. The results of the PPA study follow, showing the mean (and range) maximum systolic blood pressure in mm Hg: placebo = 121 (103 to 158); linezolid alone = 120 (107 to 135); PPA alone = 125 (106 to 139); PPA with linezolid = 147 (129 to 176). The results from the PSE study were similar to those in the PPA study. The mean maximum increase in systolic blood pressure over baseline was 32 mm Hg (range: 20 to 52 mm Hg) and 38 mm Hg (range: 18 to 79 mm Hg) during co-administration of linezolid with pseudoephedrine or phenylpropanolamine, respectively.
Serotonergic Agents
Dextromethorphan: The potential drug-drug interaction with dextromethorphan was studied in healthy volunteers. Subjects were administered dextromethorphan (two 20-mg doses given 4 hours apart) with or without linezolid. No serotonin syndrome effects (confusion, delirium, restlessness, tremors, blushing, diaphoresis, hyperpyrexia) have been observed in normal subjects receiving linezolid and dextromethorphan.
12.4 Microbiology
Mechanism of Action
Linezolid is a synthetic antibacterial agent of the oxazolidinone class, which has clinical utility in the treatment of infections caused by aerobic Gram- positive bacteria. The in vitro spectrum of activity of linezolid also includes certain Gram-negative bacteria and anaerobic bacteria. Linezolid binds to a site on the bacterial 23S ribosomal RNA of the 50S subunit and prevents the formation of a functional 70S initiation complex, which is essential for bacterial reproduction. The results of time-kill studies have shown linezolid to be bacteriostatic against enterococci and staphylococci. For streptococci, linezolid was found to be bactericidal for the majority of isolates.
Resistance
In vitro studies have shown that point mutations in the 23S rRNA are associated with linezolid resistance. Reports of vancomycin-resistant Enterococcus faecium becoming resistant to linezolid during its clinical use have been published. There are reports of Staphylococcus aureus (methicillin- resistant) developing resistance to linezolid during clinical use. The linezolid resistance in these organisms is associated with a point mutation in the 23S rRNA (substitution of thymine for guanine at position 2,576) of the organism. Organisms resistant to oxazolidinones via mutations in chromosomal genes encoding 23S rRNA or ribosomal proteins (L3 and L4) are generally cross- resistant to linezolid. Also linezolid resistance in staphylococci mediated by the enzyme methyltransferase has been reported. This resistance is mediated by the cfr (chloramphenicol-florfenicol) gene located on a plasmid which is transferable between staphylococci.
Interaction with Other Antimicrobial Drugs
In vitro studies have demonstrated additivity or indifference between linezolid and vancomycin, gentamicin, rifampin, imipenem-cilastatin, aztreonam, ampicillin, or streptomycin.
Linezolid has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].
Gram-positive bacteria
Enterococcus faecium (vancomycin-resistant isolates only)
Staphylococcus aureus (including methicillin-resistant isolates)
Streptococcus agalactiae
Streptococcus pneumoniae
Streptococcus pyogenes
The following in vitro data are available, but their clinical significance is unknown. Greater than 90% of the following bacteria exhibit an in vitro MIC less than or equal to the linezolid-susceptible breakpoint for organisms of similar genus. The safety and effectiveness of linezolid in treating clinical infections due to these bacteria have not been established in adequate and well-controlled clinical trials.
Gram-positive bacteria
Enterococcus faecalis (including vancomycin-resistant isolates)
Enterococcus faecium (vancomycin-susceptible isolates)
Staphylococcus epidermidis (including methicillin-resistant isolates)
Staphylococcus haemolyticus
Viridans group streptococci
Gram-negative bacteria
Pasteurella multocida
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria
and associated test methods and quality control standards recognized by FDA
for this drug, please see: https://www.fda.gov/STIC.
