Everolimus
These highlights do not include all the information needed to use EVEROLIMUS TABLETS safely and effectively. See full prescribing information for EVEROLIMUS TABLETS. EVEROLIMUS tablets, for oral use Initial U.S. Approval: 2009
e3dbbc24-7e55-4069-a305-e2cded2db641
HUMAN PRESCRIPTION DRUG LABEL
Jun 8, 2023
Natco Pharma Limited
DUNS: 650224736
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Everolimus
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Everolimus
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Everolimus
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Everolimus
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Hormone Receptor-Positive, HER2-Negative Breast Cancer
A randomized, double-blind, multicenter study (BOLERO-2, NCT00863655) of
everolimus tablets in combination with exemestane vs. placebo in combination
with exemestane was conducted in 724 postmenopausal women with estrogen
receptor-positive, HER2-negative advanced breast cancer with recurrence or
progression following prior therapy with letrozole or anastrozole.
Randomization was stratified by documented sensitivity to prior hormonal
therapy (yes vs. no) and by the presence of visceral metastasis (yes vs. no).
Sensitivity to prior hormonal therapy was defined as either (1) documented
clinical benefit (complete response [CR], partial response [PR], stable
disease ≥ 24 weeks) to at least one prior hormonal therapy in the advanced
setting or (2) at least 24 months of adjuvant hormonal therapy prior to
recurrence. Patients were permitted to have received 0-1 prior lines of
chemotherapy for advanced disease. The major efficacy outcome measure was
progression-free survival (PFS) evaluated by RECIST (Response Evaluation
Criteria in Solid Tumors), based on investigator (local radiology) assessment.
Other outcome measures included overall survival (OS) and objective response
rate (ORR).
Patients were randomized 2:1 to everolimus tablets 10 mg orally once daily in
combination with exemestane 25 mg once daily (n = 485) or to placebo in
combination with exemestane 25 mg orally once daily (n = 239). The two
treatment groups were generally balanced with respect to baseline demographics
and disease characteristics. Patients were not permitted to cross over to
everolimus tablets at the time of disease progression.
The trial demonstrated a statistically significant improvement in PFS by
investigator assessment (Table 20 and Figure 1). The results of the PFS
analysis based on independent central radiological assessment were consistent
with the investigator assessment. PFS results were also consistent across the
subgroups of age, race, presence and extent of visceral metastases, and
sensitivity to prior hormonal therapy.
ORR was higher in the everolimus tablets in combination with exemestane arm
vs. the placebo in combination with exemestane arm (Table 20). There were 3
complete responses (0.6%) and 58 partial responses (12%) in the everolimus
tablets arm. There were no complete responses and 4 partial responses (1.7%)
in the placebo in combination with exemestane arm.
After a median follow-up of 39.3 months, there was no statistically
significant difference in OS between the everolimus tablets in combination
with exemestane arm and the placebo in combination with exemestane arm [HR 0.89 (95% CI: 0.73, 1.10)].
Table 20: Efficacy Results in Hormone-Receptor Positive, HER-2 Negative Breast Cancer in BOLERO-2
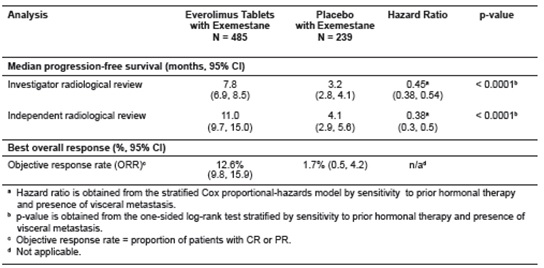
Figure 1: Kaplan-Meier Curves for Progression-Free Survival by Investigator
Radiological Review in Hormone
** Receptor-Positive, HER-2 Negative Breast Cancer in BOLERO-2**
14.2 Neuroendocrine Tumors (NET)
ancreatic Neuroendocrine Tumors (PNET)
A randomized, double-blind, multi-center trial (RADIANT-3, NCT00510068) of
everolimus tablets in combination with best supportive care (BSC) compared to
placebo in combination with BSC was conducted in patients with locally
advanced or metastatic advanced PNET and disease progression within the prior
12 months. Patients were stratified by prior cytotoxic chemotherapy (yes vs.
no) and WHO performance status (0 vs. 1 and 2). Treatment with somatostatin
analogs was allowed as part of BSC. The major efficacy outcome was PFS
evaluated by RECIST. After documented radiological progression, patients
randomized to placebo could receive open-label everolimus tablets. Other
outcome measures included ORR, response duration, and OS.
Patients were randomized 1:1 to receive either everolimus tablets 10 mg once
daily (n = 207) or placebo (n = 203).Demographics were well balanced (median
age 58 years, 55% male, 79% White). Of the 203 patients randomized to BSC, 172
patients (85%) received everolimus tablets following documented radiologic
progression.
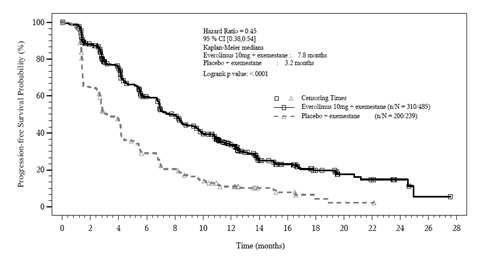
The trial demonstrated a statistically significant improvement in PFS (Table
21 and Figure 2). PFS improvement was observed across all patient subgroups,
irrespective of prior somatostatin analog use. The PFS results by investigator
radiological review, central radiological review and adjudicated radiological
review are shown below in Table 21.
Table 21: Progression-Free Survival Results in PNET in RADIANT-3
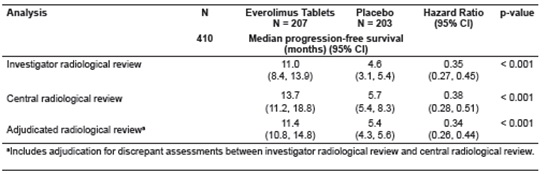
Figure 2: Kaplan-Meier Curves for Progression-Free Survival by Investigator Radiological Review in PNET in RADIANT-3
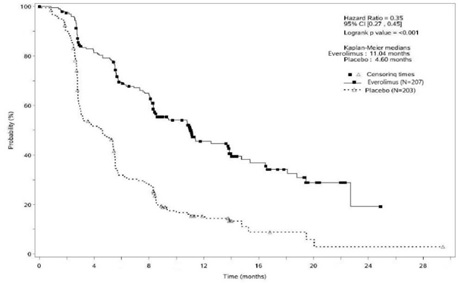
Investigator-determined response rate was 4.8% in the everolimus tablets
arm and there were no complete responses. Overall Survival (OS) was not
statistically significantly different between arms [HR = 0.94 (95% CI 0.73, 1.20); p = 0.30].
NET of Gastrointestinal (GI) or Lung Origin
A randomized, double-blind, multicenter study (RADIANT-4, NCT01524783) of
everolimus tablets in combination with BSC compared to placebo in combination
with BSC was conducted in patients with unresectable, locally advanced or
metastatic, well differentiated, non-functional NET of GI (excluding
pancreatic) or lung origin. The study required that patients had well-
differentiated (low or intermediate grade) histology, no prior or current
history of carcinoid symptoms, and evidence of disease progression within 6
months prior to randomization. Patients were randomized 2:1 to receive either
everolimus tablets 10 mg once daily or placebo, and stratified by prior
somatostatin analog use (yes vs. no), tumor origin and WHO performance status
(0 vs. 1). The major efficacy outcome measure was PFS based on independent
radiological assessment evaluated by RECIST. Additional efficacy outcome
measures were OS and ORR.
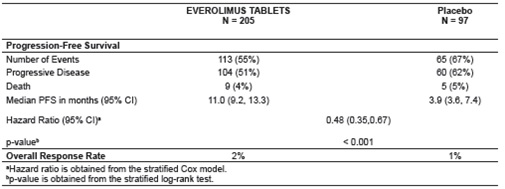
A total of 302 patients were randomized, 205 to the everolimus tablets arm and
97 to the placebo arm. The median age was 63 years (22 to 86 years); 47% were
male; 76% were White; 74% had WHO performance status of 0 and 26% had WHO
performance status of 1. The most common primary sites of tumor were lung
(30%), ileum (24%), and rectum (13%).
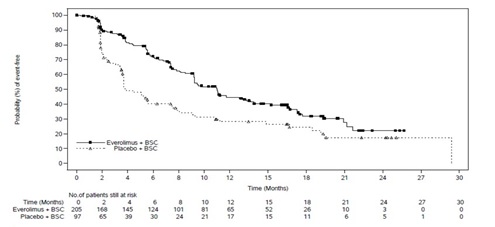
The study demonstrated a statistically significant improvement in PFS per
independent radiological review (Table 22 and Figure 3). The final OS analysis
did not show a statistically significant difference between those patients who
received everolimus tablets or placebo (HR = 0.90 [95% CI: 0.66, 1.24]).
Table 22: Progression-Free Survival in Neuroendocrine Tumors of
Gastrointestinal or Lung Origin in RADIANT-4
** Figure 3: Kaplan-Meier Curves for Progression-Free Survival in NET of GI or Lung Origin in RADIANT-4**
Lack of Efficacy in Locally Advanced or Metastatic Functional Carcinoid Tumors
The safety and effectiveness of everolimus tablets in patients with locally
advanced or metastatic functional carcinoid tumors have not been demonstrated.
In a randomized (1:1), double-blind, multi-center trial (RADIANT-2,
NCT00412061) in 429 patients with carcinoid tumors, everolimus tablets in
combination with long-acting octreotide (Sandostatin LAR®) was compared to
placebo in combination with long-acting octreotide. After documented
radiological progression, patients on the placebo arm could receive everolimus
tablets; of those randomized to placebo, 67% received open-label everolimus
tablets in combination with long-acting octreotide. The study did not meet its
major efficacy outcome measure of a statistically significant improvement in
PFS and the final analysis of OS favored the placebo in combination with long-
acting octreotide arm.
14.3 Renal Cell Carcinoma (RCC)
An international, multi-center, randomized, double-blind trial (RECORD-1, NCT00410124) comparing everolimus tablets 10 mg once daily and placebo, both in conjunction with BSC, was conducted in patients with metastatic RCC whose disease had progressed despite prior treatment with sunitinib, sorafenib, or both sequentially. Prior therapy with bevacizumab, interleukin 2, or interferon-α was also permitted. Randomization was stratified according to prognostic score and prior anticancer therapy. The major efficacy outcome measure for the trial was PFS evaluated by RECIST, based on a blinded, independent, central radiologic review. After documented radiological progression, patients randomized to placebo could receive open-label everolimus tablets. Other outcome measures included OS.
In total, 416 patients were randomized 2:1 to receive everolimus tablets (n = 277) or placebo (n = 139). Demographics were well balanced between the arms (median age 61 years; 77% male, 88% White, 74% received prior sunitinib or sorafenib, and 26% received both sequentially).
Everolimus tablets were superior to placebo for PFS (Table 23 and Figure 4). The treatment effect was similar across prognostic scores and prior sorafenib and/or sunitinib. Final OS results yield a hazard ratio of 0.90 (95% CI: 0.71, 1.14), with no statistically significant difference between the arms. Planned cross-over from placebo due to disease progression to open-label everolimus tablets occurred in 80% of the 139 patients and may have confounded the OS benefit.
Table 23: Progression-Free Survival and Objective Response Rate by Central Radiologic Review in RCC in RECORD-1
|
a Log-rank test stratified by prognostic score. | ||||
|
Everolimus N=277 |
Placebo N=139 |
Hazard Ratio (95% CI) |
p-value****a | |
|
Median Progression-free Survival (95% CI) |
4.9 months (4.0 to 5.5) |
1.9 months (1.8 to 1.9) |
0.33 (0.25 to 0.43) |
<0.0001 |
|
Objective Response Rate |
2% |
0% |
n/ab |
n/ab |
Figure 4: Kaplan-Meier Curves for Progression-Free Survival in RCC in RECORD-1

14.4 Tuberous Sclerosis Complex (TSC)-Associated Renal Angiomyolipoma
A randomized (2:1), double-blind, placebo-controlled trial (EXIST-2, NCT00790400) of everolimus tablets was conducted in 118 patients with renal angiomyolipoma as a feature of TSC (n = 113) or sporadic lymphangioleiomyomatosis (n = 5). The key eligibility requirements for this trial were at least one angiomyolipoma of ≥ 3 cm in longest diameter on CT/MRI based on local radiology assessment, no immediate indication for surgery, and age ≥ 18 years. Patients received everolimus tablets 10 mg or matching placebo orally once daily until disease progression or unacceptable toxicity. CT or MRI scans for disease assessment were obtained at baseline, 12, 24, and 48 weeks and annually thereafter. Clinical and photographic assessment of skin lesions were conducted at baseline and every 12 weeks thereafter until treatment discontinuation. The major efficacy outcome measure was angiomyolipoma response rate based on independent central radiology review, which was defined as a ≥ 50% reduction in angiomyolipoma volume, absence of new angiomyolipoma lesion ≥ 1 cm, absence of kidney volume increase ≥ 20%, and no angiomyolipoma related bleeding of ≥ Grade 2. Key supportive efficacy outcome measures were time to angiomyolipoma progression and skin lesion response rate. The primary analyses of efficacy outcome measures were limited to the blinded treatment period and conducted 6 months after the last patient was randomized. The comparative angiomyolipoma response rate analysis was stratified by use of enzyme-inducing antiepileptic drugs (EIAEDs) at randomization (yes vs. no).
Of the 118 patients enrolled, 79 were randomized to everolimus tablets and 39 to placebo. The median age was 31 years (18 to 61 years), 34% were male, and 89% were White. At baseline, 17% of patients were receiving EIAEDs. On central radiology review at baseline, 92% of patients had at least 1 angiomyolipoma of ≥ 3 cm in longest diameter, 29% had angiomyolipomas ≥ 8 cm, 78% had bilateral angiomyolipomas, and 97% had skin lesions. The median values for the sum of all target renal angiomyolipoma lesions at baseline were 85 cm3 (9 to 1612 cm3) and 120 cm3 (3 to 4520 cm3) in the everolimus tablets and placebo arms, respectively. Forty-six (39%) patients had prior renal embolization or nephrectomy. The median duration of follow-up was 8.3 months (0.7 to 24.8 months) at the time of the primary analysis.
The renal angiomyolipoma response rate was statistically significantly higher
in everolimus tablets-treated patients (Table 24). The median response
duration was 5.3+ months (2.3+ to 19.6+ months).
There were 3 patients in the everolimus tablets arm and 8 patients in the
placebo arm with documented angiomyolipoma progression by central radiologic
review (defined as a ≥ 25% increase from nadir in the sum of angiomyolipoma
target lesion volumes to a value greater than baseline, appearance of a new
angiomyolipoma ≥ 1 cm in longest diameter, an increase in renal volume ≥ 20%
from nadir for either kidney and to a value greater than baseline, or Grade ≥
2 angiomyolipoma-related bleeding). The time to angiomyolipoma progression was
statistically significantly longer in the everolimus tablets arm (HR 0.08 [95% CI: 0.02, 0.37]; p < 0.0001).
Table 24: Angiomyolipoma Response Rate in TSC-Associated Renal Angiomyolipoma in EXIST-2
|
a Per independent central radiology review | |||
|
Everolimus Tablets N=79 |
Placebo |
p-value | |
|
Primary Analysis | |||
|
Angiomyolipoma response ratea - % |
41.8 |
0 |
<0.0001 |
|
95% CI |
(30.8, 53.4) |
(0.0, 9.0) |
Skin lesion response rates were assessed by local investigators for 77 patients in the everolimus tablets arm and 37 patients in the placebo arm who presented with skin lesions at study entry. The skin lesion response rate was statistically significantly higher in the everolimus tablets arm (26% vs. 0, p = 0.0011); all skin lesion responses were partial responses, defined as visual improvement in 50% to 99% of all skin lesions durable for at least 8 weeks (Physician's Global Assessment of Clinical Condition).
Patients randomized to placebo were permitted to receive everolimus tablets at the time of angiomyolipoma progression or after the time of the primary analysis. After the primary analysis, patients treated with everolimus tablets underwent additional follow-up CT or MRI scans to assess tumor status until discontinuation of treatment or completion of 4 years of follow-up after the last patient was randomized. A total of 112 patients (79 randomized to everolimus tablets and 33 randomized to placebo) received at least one dose of everolimus tablets. The median duration of everolimus tablets treatment was 3.9 years (0.5 months to 5.3 years) and the median duration of follow-up was 3.9 years (0.9 months to 5.4 years). During the follow-up period after the primary analysis, 32 patients (in addition to the 33 patients identified at the time of the primary analysis) had an angiomyolipoma response based upon independent central radiology review. Among the 65 responders out of 112 patients, the median time to angiomyolipoma response was 2.9 months (2.6 to 33.8 months). Fourteen percent of the 112 patients treated with everolimus tablets had angiomyolipoma progression by the end of the follow-up period. No patient underwent a nephrectomy for angiomyolipoma progression and one patient underwent renal embolization while treated with everolimus tablets.
14.5 Tuberous Sclerosis Complex (TSC)-Associated Subependymal Giant Cell
Astrocytoma (SEGA)
EXIST-1
A randomized (2:1), double-blind, placebo-controlled trial (EXIST-1, NCT00789828) of everolimus tablets was conducted in 117 pediatric and adult patients with SEGA and TSC. Eligible patients had at least one SEGA lesion ≥ 1 cm in longest diameter on MRI based on local radiology assessment and one or more of the following: serial radiological evidence of SEGA growth, a new SEGA lesion ≥ 1 cm in longest diameter, or new or worsening hydrocephalus. Patients randomized to the treatment arm received everolimus tablets at a starting dose of 4.5 mg/m2 daily, with subsequent dose adjustments as needed to achieve and maintain everolimus trough concentrations of 5 to 15 ng/mL as tolerated. Everolimus tablets or matched placebo continued until disease progression or unacceptable toxicity. MRI scans for disease assessment were obtained at baseline, 12, 24, and 48 weeks, and annually thereafter.
The main efficacy outcome measure was SEGA response rate based on independent central radiology review. SEGA response was defined as a ≥ 50% reduction in the sum of SEGA volume relative to baseline, in the absence of unequivocal worsening of non-target SEGA lesions, a new SEGA lesion ≥ 1 cm, and new or worsening hydrocephalus. The primary analysis of SEGA response rate was limited to the blinded treatment period and conducted 6 months after the last patient was randomized. The analysis of SEGA response rate was stratified by use of enzyme-inducing antiepileptic drugs (EIAEDs) at randomization (yes vs. no).
Of the 117 patients enrolled, 78 were randomized to everolimus tablets and 39 to placebo. The median age was 9.5 years (0.8 to 26 years); a total of 20 patients were < 3 years, 54 patients were 3 to < 12 years, 27 patients were 12 to < 18 years, and 16 patients were ≥ 18 years; 57% were male, and 93% were White. At baseline, 18% of patients were receiving EIAEDs. Based on central radiology review at baseline, 98% of patients had at least one SEGA lesion ≥ 1.0 cm in longest diameter, 79% had bilateral SEGAs, 43% had ≥ 2 target SEGA lesions, 26% had growth in or into the inferior surface of the ventricle, 9% had evidence of growth beyond the subependymal tissue adjacent to the ventricle, and 7% had radiographic evidence of hydrocephalus. The median values for the sum of all target SEGA lesions at baseline were 1.63 cm3 (0.18 to 25.15 cm3) and 1.30 cm3 (0.32 to 9.75 cm3) in the everolimus tablets and placebo arms respectively. Eight (7%) patients had prior SEGA-related surgery. The median duration of follow-up was 8.4 months (4.6 to 17.2 months) at the time of primary analysis.
The SEGA response rate was statistically significantly higher in everolimus tablets-treated patients (Table 25). At the time of the primary analysis, all SEGA responses were ongoing and the median duration of response was 5.3 months (2.1 to 8.4 months).
With a median follow-up of 8.4 months, SEGA progression was detected in 15.4% of the 39 patients randomized to receive placebo and none of the 78 patients randomized to receive everolimus tablets. No patient in either treatment arm required surgical intervention.
Table 25: Subependymal Giant Cell Astrocytoma Response Rate in TSC- Associated SEGA in EXIST-1
|
a Per independent central radiology review | |||
|
Everolimus Tablets |
Placebo N=39 |
p-value | |
|
Final analysis | |||
|
SEGA response ratea - (%) |
35 |
0 |
<0.0001 |
|
95% CI |
24, 46 |
0, 9 |
Patients randomized to placebo were permitted to receive everolimus tablets at the time of SEGA progression or after the primary analysis, whichever occurred first. After the primary analysis, patients treated with everolimus tablets underwent additional follow-up MRI scans to assess tumor status until discontinuation of treatment or completion of 4 years of follow-up after the last patient was randomized. A total of 111 patients (78 patients randomized to everolimus tablets and 33 patients randomized to placebo) received at least one dose of everolimus tablets. Median duration of everolimus tablets treatment and follow-up was 3.9 years (0.2 to 4.9 years).
By four years after the last patient was enrolled, 58% of the 111 patients treated with everolimus tablets had a ≥ 50% reduction in SEGA volume relative to baseline, including 27 patients identified at the time of the primary analysis and 37 patients with a SEGA response after the primary analysis. The median time to SEGA response was 5.3 months (2.5 to 33.1 months). Twelve percent of the 111 patients treated with everolimus tablets had documented disease progression by the end of the follow-up period and no patient required surgical intervention for SEGA during the study.
Study 2485
Study 2485 (NCT00411619) was an open-label, single-arm trial conducted to evaluate the antitumor activity of everolimus tablets 3 mg/m2/orally once daily in patients with SEGA and TSC. Serial radiological evidence of SEGA growth was required for entry. Tumor assessments were performed every 6 months for 60 months after the last patient was enrolled or disease progression, whichever occurred earlier. The major efficacy outcome measure was the reduction in volume of the largest SEGA lesion with 6 months of treatment, as assessed via independent central radiology review. Progression was defined as an increase in volume of the largest SEGA lesion over baseline that was ≥ 25% over the nadir observed on study.
A total of 28 patients received everolimus tablets for a median duration of
5.7 years (5 months to 6.9 years); 82% of the 28 patients remained on
everolimus tablets for at least 5 years. The median age was 11 years (3 to 34
years), 61% male, 86% White.
At the primary analysis, 32% of the 28 patients (95% CI: 16%, 52%) had an
objective response at 6 months, defined as at least a 50% decrease in volume
of the largest SEGA lesion. At the completion of the study, the median
duration of durable response was 12 months (3 months to 6.3 years).
By 60 months after the last patient was enrolled, 11% of the 28 patients had documented disease progression. No patient developed a new SEGA lesion while on everolimus tablets. Nine additional patients were identified as having a ≥50% volumetric reduction in their largest SEGA lesion between 1 to 4 years after initiating everolimus tablets including 3 patients who had surgical resection with subsequent regrowth prior to receiving everolimus tablets.
