Trelstar
These highlights do not include all the information needed to use TRELSTAR safely and effectively. See full prescribing information for TRELSTAR. TRELSTAR (triptorelin pamoate for injectable suspension), for intramuscular use Initial U.S. Approval: 2000
b1b84d62-a369-a4b7-5c41-dd1f553a18f3
HUMAN PRESCRIPTION DRUG LABEL
Nov 22, 2023
Verity Pharmaceuticals Inc.
DUNS: 117448813
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
triptorelin pamoate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
triptorelin pamoate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
triptorelin pamoate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
Drug Labeling Information
USE IN SPECIFIC POPULATIONS SECTION
8****USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animal studies and mechanism of action, TRELSTAR can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. Expected hormonal changes that occur with TRELSTAR treatment increase the risk for pregnancy loss. In animal developmental and reproductive toxicology studies, daily administration of triptorelin to pregnant rats during the period of organogenesis caused maternal toxicity and embryo-fetal toxicities, including loss of pregnancy, at doses as low as 0.2, 0.8, and 8 times the estimated human daily dose based on body surface area. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus.
Data
Animal Data
Studies in pregnant rats administered triptorelin at doses of 2, 10, and 100 mcg/kg/day (approximately equivalent to 0.2, 0.8, and 8 times the estimated human daily dose based on body surface area) during the period of organogenesis demonstrated maternal toxicity and embryo-fetal toxicities. Embryo-fetal toxicities consisted of pre-implantation loss, increased resorption, and reduced mean number of viable fetuses at the high dose. Teratogenic effects were not observed in viable fetuses in rats or mice. Doses administered to mice were 2, 20, and 200 mcg/kg/day (approximately equivalent to 0.1, 0.7, and 7 times the estimated human daily dose based on body surface area).
8.2 Lactation
The safety and efficacy of TRELSTAR have not been established in females. There are no data on the presence of triptorelin in human milk, the effects of the drug on milk production, or the effects of the drug on the breastfed child. Because of the potential for serious adverse reactions in a breastfed child from TRELSTAR, a decision should be made to either discontinue breastfeeding, or discontinue the drug taking into account the importance of the drug to the mother.
8.3 Females and Males of Reproductive Potential
Infertility
Males
Based on mechanism of action, TRELSTAR may impair fertility in males of reproductive potential [see Clinical Pharmacology (12.1)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Prostate cancer occurs primarily in an older population. Clinical studies with TRELSTAR have been conducted primarily in patients ≥ 65 years [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
8.6 Renal Impairment
Subjects with renal impairment had higher exposure than young healthy males [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Subjects with hepatic impairment had higher exposure than young healthy males [see Clinical Pharmacology (12.3)].
- Females and males of reproductive potential: TRELSTAR may impair fertility. (8.3)
DESCRIPTION SECTION
11****DESCRIPTION
TRELSTAR is a white to slightly yellow lyophilized cake. When reconstituted, TRELSTAR has a milky appearance. It contains a pamoate salt of triptorelin, a synthetic decapeptide agonist analog of gonadotropin releasing hormone (GnRH). The chemical name of triptorelin pamoate is 5-oxo-L-prolyl-L-histidyl-L- tryptophyl-L-seryl-L-tyrosyl-D-tryptophyl-L-leucyl-L-arginyl-L-prolylglycine amide (pamoate salt). The empirical formula is C64H82N18O13 · C23H16O6 and the molecular weight is 1699.9. The structural formula is:
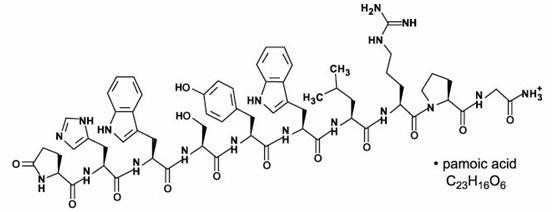
Structural formula for TRELSTAR (triptorelin pamoate).
The TRELSTAR products are sterile, lyophilized biodegradable microgranule formulations supplied as single dose vials. Refer to Table 5 for the composition of each TRELSTAR product.
Table 5. TRELSTAR Composition|
Ingredients |
TRELSTAR**** |
TRELSTAR**** |
TRELSTAR**** |
|
triptorelin pamoate |
3.75 mg |
11.25 mg |
22.5 mg |
|
poly-d,l-lactide-co-glycolide |
138 mg |
120 mg |
183 mg |
|
mannitol, USP |
71 mg |
74 mg |
74 mg |
|
carboxymethylcellulose sodium, USP |
25 mg |
26 mg |
26 mg |
|
polysorbate 80, NF |
1.7 mg |
1.7 mg |
1.7 mg |
When 2 mL sterile water is added to the vial containing TRELSTAR and mixed, a suspension is formed which is intended as an intramuscular injection. TRELSTAR is available in a vial plus a MIXJECT vial adapter, and a separate pre-filled syringe that contains sterile water for injection, USP, 2 mL.
NONCLINICAL TOXICOLOGY SECTION
13****NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In rats, triptorelin doses of 120, 600, and 3000 mcg/kg given every 28 days (approximately 0.3, 2, and 8 times the human monthly dose based on body surface area) resulted in increased mortality with a drug treatment period of 13 – 19 months. The incidences of benign and malignant pituitary tumors and histiosarcomas were increased in a dose-related manner. No oncogenic effect was observed in mice administered triptorelin for 18 months at doses up to 6000 mcg/kg every 28 days (approximately 8 times the human monthly dose based on body surface area).
Mutagenicity studies performed with triptorelin using bacterial and mammalian systems (in vitro Ames test and chromosomal aberration test in CHO cells and an in vivo mouse micronucleus test) provided no evidence of mutagenic potential.
After 60 days of subcutaneous treatment followed by a minimum of four estrus cycles prior to mating, triptorelin, at doses of 2, 20, and 200 mcg/kg/day in saline (approximately 0.2, 2, and 16 times the estimated human daily dose based on body surface area) or 2 monthly injections as slow release microspheres (~20 mcg/kg/day), had no effect on the fertility or general reproductive function of female rats.
No studies were conducted to assess the effect of triptorelin on male fertility.
