Ativan
89057c93-8155-4040-acec-64e877bd2b4c
HUMAN PRESCRIPTION DRUG LABEL
Jan 13, 2023
Bausch Health US LLC
DUNS: 831922468
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
lorazepam
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
lorazepam
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
lorazepam
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel -.5 mg Bottle Label
NDC0187-0063-01
Rx only
Ativan**®**
(lorazepam) Tablets
CIV
SEALED
FOR YOUR
PROTECTION
0.5 mg
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
100 Tablets
BAUSCH Health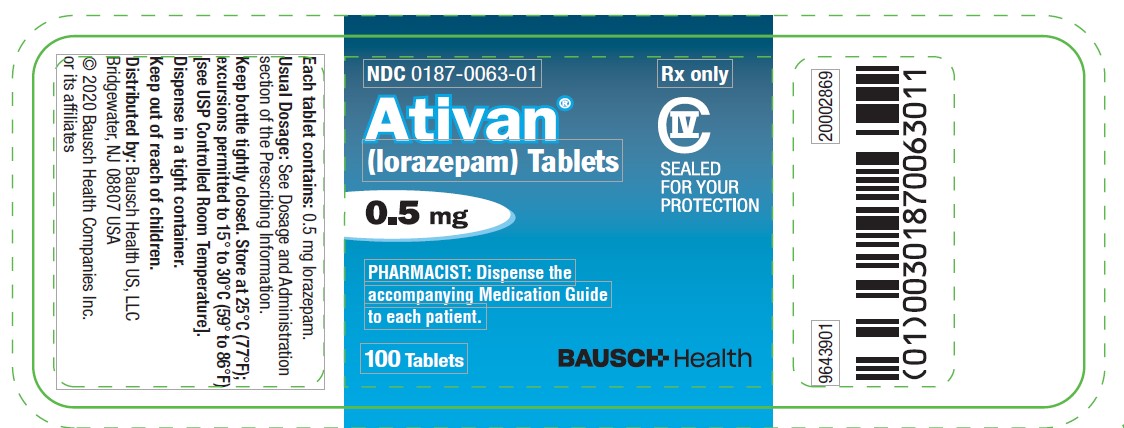
HOW SUPPLIED SECTION
HOW SUPPLIED
Ativan® (lorazepam) Tablets are available in the following dosage strengths:
0.5 mg, white, five-sided (shield shape) tablet with a raised "A" on one side and "BPI" and "63" impressed on reverse side.
NDC 0187-0063-01 - Bottles of 100 tablets.
1 mg, white, five-sided (shield shape) tablet with a raised "A" on one side and "BPI" and "64" impressed on scored reverse side.
NDC 0187-0064-01 - Bottles of 100 tablets.
NDC 0187-0064-10 - Bottles of 1,000 tablets.
2 mg, white, five-sided (regular pentagon) tablet with a raised "A" and impressed "2" on one side and "BPI" and "65" impressed on scored reverse side.
NDC 0187-0065-01 - Bottles of 100 tablets.
Keep bottles tightly closed.
Keep out of reach of children.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Dispense in a tight container.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Steinbach, MB R5G 1Z7, Canada
Ativan is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2023 Bausch Health Companies Inc. or its affiliates
9644303 Rev. 01/2023
SPL MEDGUIDE SECTION
Medication Guide
|
MEDICATION GUIDE ATIVAN (AT-ivan) (lorazepam) Tablets
| |
|
What is the most important information I should know about ATIVAN? • • • • • • • • • • • • • • • | |
|
What is ATIVAN? • • • • • • | |
|
Do not take ATIVAN if you: • | |
|
Before you take ATIVAN, tell your healthcare provider about all of your medical conditions, including if you: • • • • • • • • • • • • Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking ATIVAN with certain other medicines can cause side effects or affect how well ATIVAN or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider. | |
|
How should I take ATIVAN? • • | |
|
What are the possible side effects of ATIVAN? ATIVAN may cause serious side effects, including: • • • • • | |
|
The most common side effects of ATIVAN include: | |
|
• sedation • dizziness • weakness • unsteadiness These are not all the possible side effects of ATIVAN. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
|
How should I store ATIVAN? • • | |
|
General information about the safe and effective use of ATIVAN Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ATIVAN for a condition for which it was not prescribed. Do not give ATIVAN to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ATIVAN that is written for health professionals. | |
|
What are the ingredients in ATIVAN? **Active ingredient:**lorazepam **Inactive ingredients:**lactose monohydrate, magnesium stearate, microcrystalline cellulose, and polacrilin potassium. Distributed by: Bausch Health US, LLC **Manufactured by:**Bausch Health Companies Inc. ATIVAN is a trademark of Bausch Health Companies Inc. or its affiliates. For more information, call 1-800-321-4576. © 2023 Bausch Health Companies Inc. or its affiliates | |
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. | |
|
Revised: 01/2023 |
9644303 |

