Droxidopa
These highlights do not include all the information needed to use DROXIDOPA CAPSULES safely and effectively. See full prescribing information for DROXIDOPA CAPSULES. DROXIDOPA capsules, for oral use Initial U.S. Approval: 2014
700c0bc6-b089-4478-8e62-4232cad2348e
HUMAN PRESCRIPTION DRUG LABEL
Feb 21, 2019
MSN LABORATORIES PRIVATE LIMITED
DUNS: 650786952
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Droxidopa
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Droxidopa
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Droxidopa
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Studies in Neurogenic Orthostatic Hypotension
Clinical studies (described below) examined the efficacy of droxidopa in the
short-term (1 to 2 weeks) and over longer-term periods (8 weeks; 3 months).
Studies 301 and 306B showed a treatment effect of droxidopa at Week 1, but
none of the studies demonstrated continued efficacy beyond 2 weeks of
treatment.
Study 306B was a multi-center, double-blind, randomized, placebo-controlled,
parallel-group study in patients with symptomatic nOH and Parkinson’s disease.
Patients entering the study were required to have a decrease of at least 20 mm
Hg or 10 mm Hg, respectively, in systolic or diastolic blood pressure, within
3 minutes after standing, as well as symptoms associated with neurogenic
orthostatic hypotension. The study had an initial dose titration period that
lasted up to 2 weeks in which patients received placebo or 100 to 600 mg of
droxidopa three times daily, followed by an 8-week treatment period.
Efficacy was measured using the OHSA Item #1 score (“dizziness,
lightheadedness, feeling faint, and feeling like you might black out”) at Week
1, in patients who had completed titration and 1 week of maintenance therapy.
A total of 171 patients were enrolled, and 147 patients were included in the
efficacy analysis. The mean age was 72 years, and patients were mostly
Caucasian. During the study, 94% of placebo-treated patients and 88% on
droxidopa were taking dopa-decarboxylase inhibitors; 17% of placebo-treated
patients and 26% on droxidopa were taking fludrocortisone. There were more
premature discontinuations in the droxidopa group (28%) than in the placebo
group (20%).
In both groups, the mean baseline dizziness score was 5.1 on an 11-point
scale. At Week 1, patients showed a statistically significant mean 0.9 unit
decrease in dizziness with droxidopa versus placebo (P=0.028), but the effect
did not persist beyond Week 1. The data at all time points are shown in Figure
1.
Patients receiving droxidopa also had a greater increase, compared to placebo,
in the Week 1 lowest standing systolic blood pressure within 3 minutes after
standing (5.6 mm Hg; P=0.032).
Figure 1. Mean Change in OHSA Item 1 Score by Week in Study 306B
****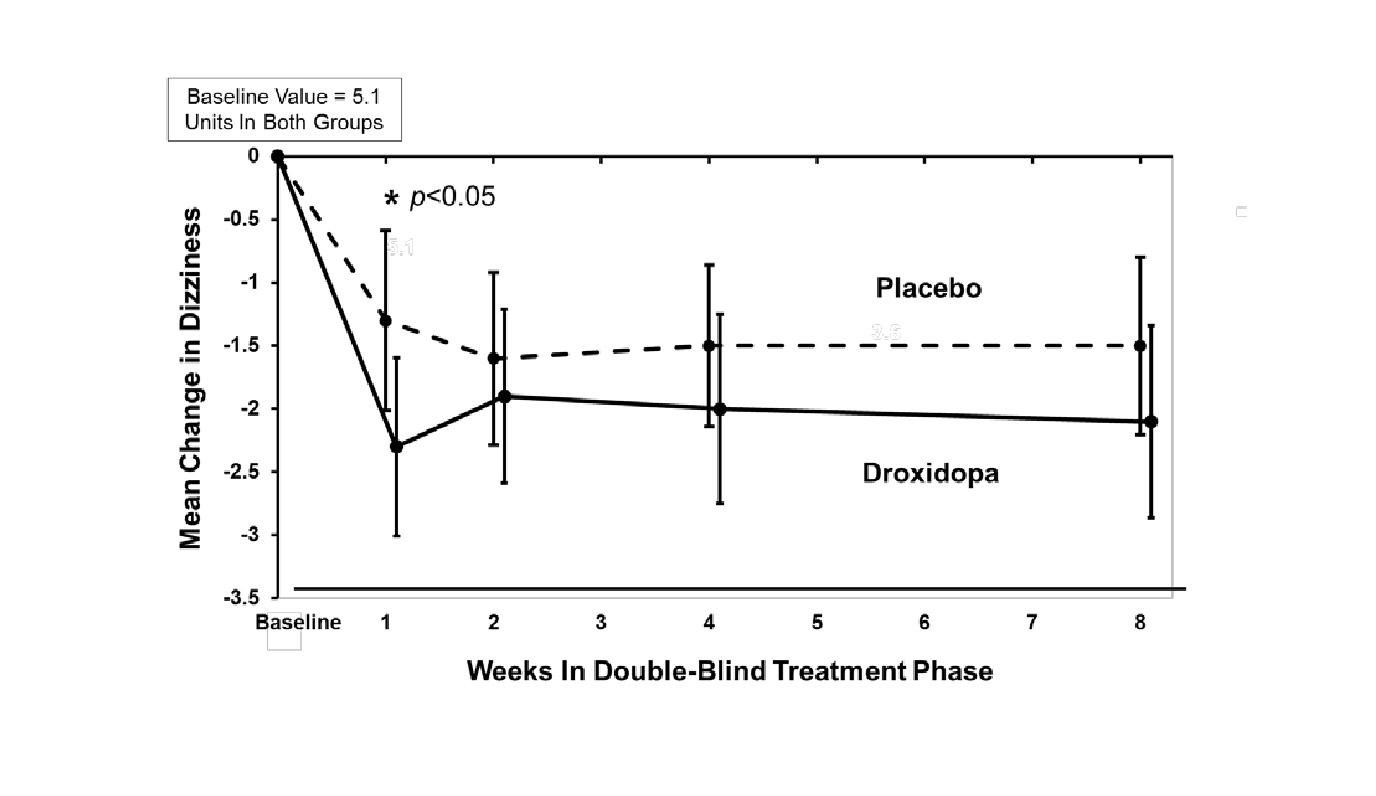
Note: The graph is based on observed data only. The error bars are the 95% confidence interval of the mean change from baseline in OHSA Item 1 scores.
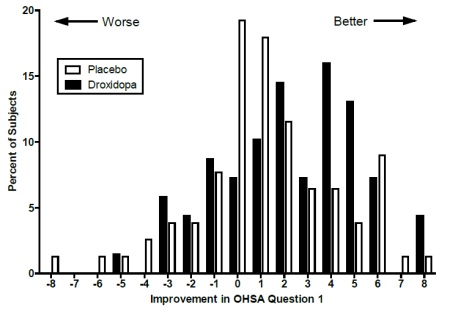
Figure 2. Distribution of Patients by Change in OHSA Item 1, Baseline to
Week 1, in Study 306B
Figure 2 shows the distribution of changes from Baseline to Week 1 in the OHSA
Item #1 score. Overall, the figure shows that patients treated with droxidopa
improved more than those treated with placebo.
Study 301 was a multicenter, multinational, double-blind, randomized, placebo-
controlled, parallel-group study in patients with symptomatic neurogenic
orthostatic hypotension. The study included an initial open-label dose
titration period, a 7-day washout period, and a randomized double-blind 7-day
treatment period. To be eligible for enrollment, patients were required to
have a decrease in systolic or diastolic blood pressure of at least 20 or 10
mm Hg, respectively, within 3 minutes after standing. The study was enriched,
such that only patients who had been identified as “responders” during the
titration period were randomized to droxidopa or placebo. To be considered a
responder, a patient had to demonstrate improvement on the OHSA Item #1 score
by at least 1 point, as well as an increase in systolic blood pressure of at
least 10 mm Hg post-standing, during the open-label dose titration period.
Patients who dropped out during the titration period because of side effects
or other reasons were also not included in the double-blind portion of the
study.
Patients had a primary diagnosis of Parkinson’s disease (n=60), pure autonomic
failure (n=36), or multiple system atrophy (n=26). The mean age was 60 years,
and most were Caucasian. 45% of patients were taking dopa decarboxylase
inhibitors, and 29% were taking fludrocortisone.
Efficacy was measured using the Orthostatic Hypotension Questionnaire (OHQ), a
patient-reported outcome that measures symptoms of nOH and their impact on the
patient’s ability to perform daily activities that require standing and
walking. The OHQ includes OHSA Item #1 as one of several components. A
statistically significant treatment effect was not demonstrated on OHQ
(treatment effect of 0.4 unit, P=0.19).
The mean baseline dizziness score on OHSA Item #1 (“dizziness,
lightheadedness, feeling faint, and feeling like you might black out”) was 5.2
units on an 11-point scale. At Week 1 of treatment, patients showed a mean 0.7
unit decrease in dizziness with droxidopa versus placebo (P=0.06).
Study 302 (n=101) was a placebo-controlled, 2-week randomized withdrawal study
of droxidopa in patients with symptomatic nOH. Study 303 (n=75) was an
extension of Studies 301 and 302, where patients received their titrated dose
of droxidopa for 3 months and then entered a 2-week randomized withdrawal
phase. Neither study showed a statistically significant difference between
treatment arms on its primary endpoint. Considering these data, the
effectiveness of droxidopa beyond 2 weeks is uncertain, and patients should be
evaluated periodically to determine whether droxidopa is continuing to provide
a benefit.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Elevations in Blood Pressure
Counsel patients that droxidopa causes elevations in blood pressure and
increases the risk of supine hypertension, which could lead to strokes, heart
attacks, and death. Instruct patients to rest and sleep in an upper- body
elevated position and monitor blood pressure. Instruct patients how to manage
observed blood pressure elevations. To reduce the risk of supine hypertension,
in addition to raising the upper body, the late afternoon dose of droxidopa
should be taken at least three hours before bedtime [ see Warnings and Precautions (5.1)].
Concomitant Treatments
Counsel patients about the concomitant use of drugs to treat other conditions
that may have an additive effect with droxidopa [ see Drug Interactions (7)].
Allergic Reactions
Counsel patients to discontinue droxidopa and seek immediate medical attention
if any signs or symptoms of a hypersensitivity reaction such as anaphylaxis,
angioedema, bronchospasm, urticaria or rash occur [ see Warnings and Precautions (5.4)].
Lactation
Advise women not to breastfeed during treatment with droxidopa [ see Use in Specific Populations (8.2)].
Food
Patients should take droxidopa the same way each time, either with food or
without food [ see Dosage and Administration (2.1)].
Missed Dose
If a dose is missed, patients should take the next dose at the regularly
scheduled time and should not double the dose.
Manufactured by:
MSN Laboratories Private Limited
Telangana – 509228,
INDIA
Distributed by:
MSN Pharmaceuticals Inc.
Piscataway, NJ 08854-3714
Issued on:
February 2019
