Rugby Famotidine
Rugby Laboratories Famotidine Drug Facts
cdb6caa5-5be6-4597-a930-b8c816ac3040
HUMAN OTC DRUG LABEL
Aug 13, 2025
Rugby Laboratories
DUNS: 079246066
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Famotidine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
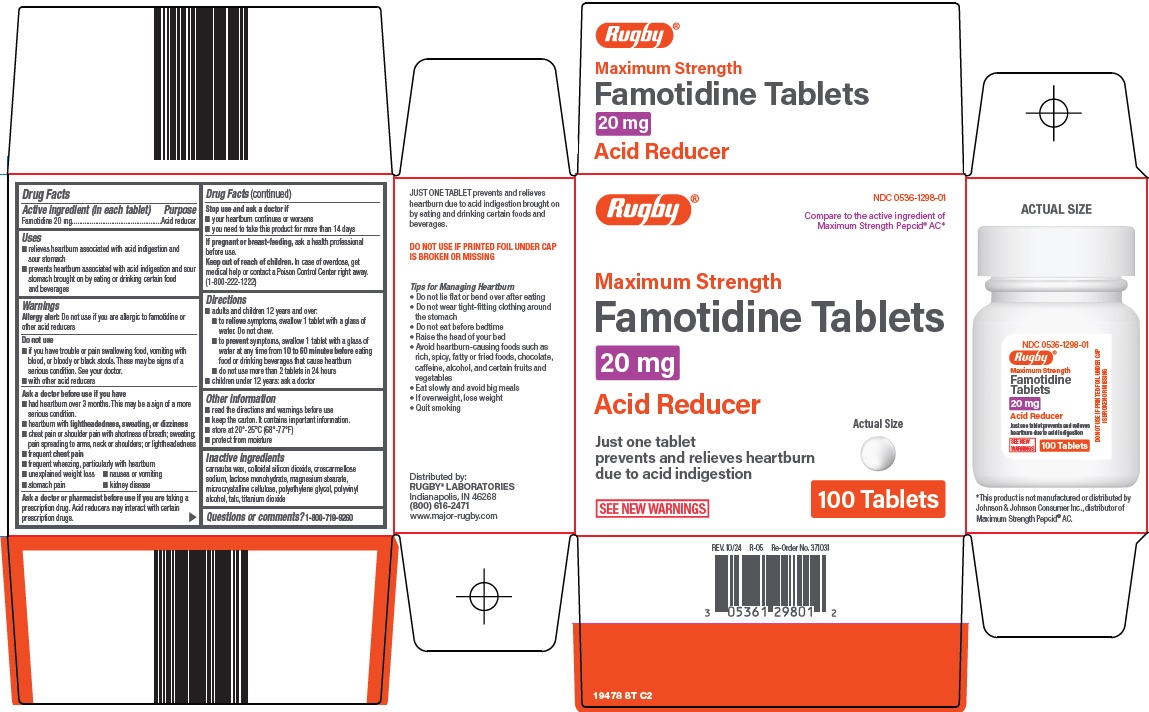
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
Rugby®
Compare to the active ingredient of Maximum Strength Pepcid® AC
Maximum Strength
Famotidine Tablets
20 mg
Acid Reducer
Actual Size
Just one tablet prevents and relieves heartburn due to acid indigestion
SEE NEW WARNINGS
100 Tablets
INDICATIONS & USAGE SECTION
Uses
•
relieves heartburn associated with acid indigestion and sour stomach
•
prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain food and beverages
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each tablet)
Famotidine 20 mg
OTC - PURPOSE SECTION
Purpose
Acid reducer
WARNINGS SECTION
Warnings
**Allergy alert:**Do not use if you are allergic to famotidine or other acid reducers
Do not use
•
if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
•
with other acid reducers
Ask a doctor before use if you have
•
had heartburn over 3 months. This may be a sign of a more serious condition.
•
heartburn with**lightheadedness, sweating, or dizziness**
•
chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
•
frequent**chest pain**
•
frequent wheezing, particularly with heartburn
•
unexplained weight loss
•
nausea or vomiting
•
stomach pain
•
kidney disease
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Acid reducers may interact with certain prescription drugs.
Stop use and ask a doctor if
•
your heartburn continues or worsens
•
you need to take this product for more than 14 days
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
DOSAGE & ADMINISTRATION SECTION
Directions
•
adults and children 12 years and over:
•
to**relieve**symptoms, swallow 1 tablet with a glass of water. Do not chew.
•
to**prevent**symptoms, swallow 1 tablet with a glass of water at any time from**10 to 60 minutes before**eating food or drinking beverages that cause heartburn
•
do not use more than 2 tablets in 24 hours
•
children under 12 years: ask a doctor
STORAGE AND HANDLING SECTION
Other information
•
read the directions and warnings before use
•
keep the carton. It contains important information.
•
store at 20°-25°C (68°-77°F)
•
protect from moisture
INACTIVE INGREDIENT SECTION
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide
OTC - QUESTIONS SECTION
Questions or comments?
1-800-719-9260
