Amikacin Sulfate
Amikacin Sulfate Injection, USP Rx only
a43188fa-f228-4acd-8a5d-9a3462034f4b
HUMAN PRESCRIPTION DRUG LABEL
Jun 18, 2025
Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
DUNS: 780779901
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Amikacin Sulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
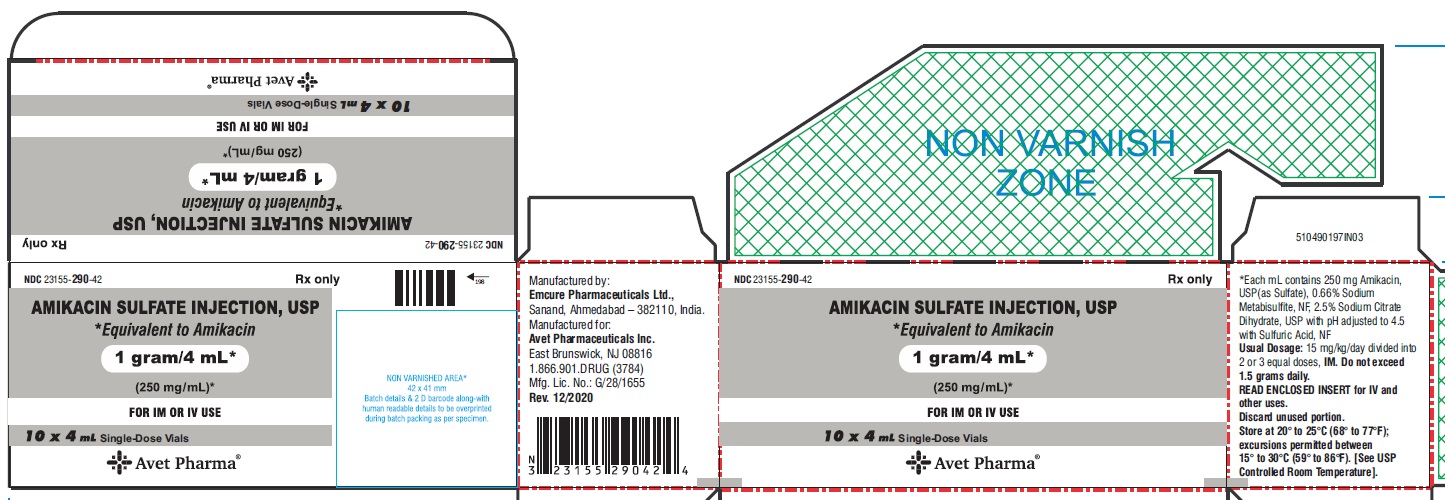
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal display panel -1 g/4 mL - carton
AMIKACIN SULFATE INJECTION, USP
*Equivalent to Amikacin
1 gram /4 mL*
(250 mg/mL)*
Rx only
NDC 23155-290-42
FOR IM OR IV USE
10 X 4 mL** Single-Dose Vials**
BOXED WARNING SECTION
WARNINGS
Patients treated with parenteral aminoglycosides should be under close clinical observation because of the potential ototoxicity and nephrotoxicity associated with their use. Safety for treatment periods which are longer than 14 days has not been established.
Neurotoxicity, manifested as vestibular and permanent bilateral auditory ototoxicity, can occur in patients with preexisting renal damage and in patients with normal renal function treated at higher doses and/or for periods longer than those recommended. The risk of aminoglycoside-induced ototoxicity is greater in patients with renal damage. High frequency deafness usually occurs first and can be detected only by audiometric testing. Vertigo may occur and may be evidence of vestibular injury. Other manifestations of neurotoxicity may include numbness, skin tingling, muscle twitching and convulsions. The risk of hearing loss due to aminoglycosides increases with the degree of exposure to either high peak or high trough serum concentrations. Patients developing cochlear damage may not have symptoms during therapy to warn them of developing eighth-nerve toxicity, and total or partial irreversible bilateral deafness may occur after the drug has been discontinued. Aminoglycoside-induced ototoxicity is usually irreversible. Aminoglycosides are potentially nephrotoxic. The risk of nephrotoxicity is greater in patients with impaired renal function and in those who receive high doses or prolonged therapy.
Neuromuscular blockade and respiratory paralysis have been reported following parenteral injection, topical instillation (as in orthopedic and abdominal irrigation or in local treatment of empyema), and following oral use of aminoglycosides. The possibility of these phenomena should be considered if aminoglycosides are administered by any route, especially in patients receiving anesthetics, neuromuscular blocking agents such as tubocurarine, succinylcholine, decamethonium, or in patients receiving massive transfusions of citrate-anticoagulated blood. If blockage occurs, calcium salts may reverse these phenomena, but mechanical respiratory assistance may be necessary.
Renal and eighth-nerve function should be closely monitored especially in patients with known or suspected renal impairment at the onset of therapy and also in those whose renal function is initially normal but who develop signs of renal dysfunction during therapy. Serum concentrations of amikacin should be monitored when feasible to assure adequate levels and to avoid potentially toxic levels and prolonged peak concentrations above 35 micrograms per mL. Urine should be examined for decreased specific gravity, increased excretion of proteins, and the presence of cells or casts. Blood urea nitrogen, serum creatinine, or creatinine clearance should be measured periodically. Serial audiograms should be obtained where feasible in patients old enough to be tested, particularly high risk patients. Evidence of ototoxicity (dizziness, vertigo, tinnitus, roaring in the ears, and hearing loss) or nephrotoxicity requires discontinuation of the drug or dosage adjustment.
Concurrent and/or sequential systemic, oral or topical use of other neurotoxic or nephrotoxic products, particularly bacitracin, cisplatin, amphotericin B, cephaloridine, paromomycin, viomycin, polymyxin B, colistin, vancomycin, or other aminoglycosides should be avoided. Other factors that may increase risk of toxicity are advanced age and dehydration.
The concurrent use of amikacin with potent diuretics (ethacrynic acid, or furosemide) should be avoided since diuretics by themselves may cause ototoxicity. In addition, when administered intravenously, diuretics may enhance aminoglycoside toxicity by altering antibiotic concentrations in serum and tissue.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Intramuscular Administration
Amikacin is rapidly absorbed after intramuscular administration. In normal adult volunteers, average peak serum concentrations of about 12, 16, and 21 mcg/mL are obtained 1 hour after intramuscular administration of 250 mg (3.7 mg/kg), 375 mg (5 mg/kg), 500 mg (7.5 mg/kg), single doses, respectively. At 10 hours, serum levels are about 0.3 mcg/mL, 1.2 mcg/mL, and 2.1 mcg/mL, respectively.
Tolerance studies in normal volunteers reveal that amikacin is well tolerated locally following repeated intramuscular dosing, and when given at maximally recommended doses, no ototoxicity or nephrotoxicity has been reported. There is no evidence of drug accumulation with repeated dosing for 10 days when administered according to recommended doses.
With normal renal function, about 91.9% of an intramuscular dose is excreted unchanged in the urine in the first 8 hours, and 98.2% within 24 hours. Mean urine concentrations for 6 hours are 563 mcg/mL following a 250 mg dose, 697 mcg/mL following a 375 mg dose, and 832 mcg/mL following a 500 mg dose.
Preliminary intramuscular studies in newborns of different weights (less than 1.5 kg, 1.5 to 2 kg, over 2 kg) at a dose of 7.5 mg/kg revealed that, like other aminoglycosides, serum half-life values were correlated inversely with post-natal age and renal clearances of amikacin. The volume of distribution indicates that amikacin, like other aminoglycosides, remains primarily in the extracellular fluid space of neonates. Repeated dosing every 12 hours in all the above groups did not demonstrate accumulation after 5 days.
Intravenous Administration
Single doses of 500 mg (7.5 mg/kg) administered to normal adults as an infusion over a period of 30 minutes produced a mean peak serum concentration of 38 mcg/mL at the end of the infusion, and levels of 24 mcg/mL, 18 mcg/mL, and 0.75 mcg/mL at 30 minutes, 1 hour, and 10 hours post-infusion, respectively. Eighty-four percent of the administered dose was excreted in the urine in 9 hours and about 94% within 24 hours. Repeat infusions of 7.5 mg/kg every 12 hours in normal adults were well tolerated and caused no drug accumulation.
General
Pharmacokinetic studies in normal adult subjects reveal the mean serum half- life to be slightly over 2 hours with a mean total apparent volume of distribution of 24 liters (28% of the body weight). By the ultrafiltration technique, reports of serum protein binding range from 0 to 11%. The mean serum clearance rate is about 100 mL/min and the renal clearance rate is 94 mL/min in subjects with normal renal function.
Amikacin is excreted primarily by glomerular filtration. Patients with impaired renal function or diminished glomerular filtration pressure excrete the drug much more slowly (effectively prolonging the serum half-life). Therefore, renal function should be monitored carefully and dosage adjusted accordingly (see suggested dosage schedule underDOSAGE AND ADMINISTRATION)
Following administration at the recommended dose, therapeutic levels are found in bone, heart, gallbladder, and lung tissue in addition to significant concentrations in urine, bile, sputum, bronchial secretions, interstitial, pleural, and synovial fluids.
Spinal fluid levels in normal infants are approximately 10 to 20% of the serum concentrations and may reach 50% when the meninges are inflamed. Amikacin has been demonstrated to cross the placental barrier and yield significant concentrations in amniotic fluid. The peak fetal serum concentration is about 16% of the peak maternal serum concentration and maternal and fetal serum half-life values are about 2 and 3.7 hours, respectively.
Microbiology
Mechanism of Action
Amikacin, an aminoglycoside, binds to the prokaryotic ribosome, inhibiting protein synthesis in susceptible bacteria. It is bactericidal in vitro against Gram-positive and Gram-negative bacteria.
Mechanism of Resistance
Aminoglycosides are known to be ineffective against Salmonella and Shigella species in patients. Therefore, in vitro susceptibility test results should not be reported.
Amikacin resists degradation by certain aminoglycoside inactivating enzymes known to affect gentamicin, tobramycin, and kanamycin.
Aminoglycosides in general have a low order of activity against Gram-positive organisms other than Staphylococcal isolates.
Interaction with Other Antimicrobials
In vitro studies have shown that amikacin sulfate combined with a beta-lactam antibiotic acts synergistically against many clinically significant Gram- negative organisms.
Antimicrobial Activity
Amikacin has been shown to be active against the following bacteria, both in vitro and in clinical infections [seeIndications and Usage]
Gram-positive Bacteria
Staphylococcus species
Gram-negative Bacteria
Pseudomonas species
Escherichia coli
Proteus species (indole-positive and indole-negative)
Klebsiella species
Enterobacter species
Serratia species
Acinetobacter species
Amikacin has demonstrated in vitro activity against the following bacteria. The safety and effectiveness of amikacin in treating clinical infections due to these bacteria have not been established in adequate and well-controlled trials.
Citrobacter freundii
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
SPL UNCLASSIFIED SECTION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of the amikacin and other antibacterial drugs, amikacin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION SECTION
DESCRIPTION
Amikacin sulfate is semi-synthetic aminoglycoside antibiotic derived from kanamycin. It is C22H43N5O13•2H2SO4•O-3-amino-3-deoxy-α-D- glucopyranosyl-(1→4)-O-[6-amino-6-deoxy-α-D- glucopyranosyl-(1→6)]-N3-(4-amino-L-2-hydroxybutyryl)-2-deoxy-L-streptamine sulfate (1:2)
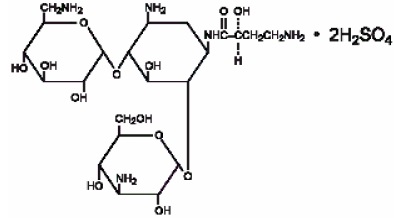
**M.W.**585.61
The dosage form is supplied as a sterile, colorless to light straw colored solution for intramuscular or intravenous use. The 500 mg per 2 mL vial and 1 gram per 4 mL vial contains per each mL: 250 mg Amikacin (as the Amikacin sulfate USP), 0.66% sodium metabisulfite as an antioxidant, 2.5% sodium citrate dihydrate as a buffering agent with pH adjusted to 4.5 with sulfuric acid.
HOW SUPPLIED SECTION
HOW SUPPLIED
Amikacin Sulfate Injection USP is supplied as a clear colorless to light straw colored solution which requires no refrigeration. At times the solution may become a very pale yellow; this does not indicate a decrease in potency.
Amikacin Sulfate Injection, USP
|
** Strength** |
** NDC** |
** Packing configuration** |
|
500 mg per 2 mL |
23155-290-41 |
10 Single-Dose Vials |
|
1 gram per 4 mL |
23155-290-42 |
10 Single-Dose Vials |
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature].
To report SUSPECTED ADVERSE EVENTS, contact Avet Pharmaceuticals Inc. at 1-866-901-DRUG (3784) or FDA at 1-800-FDA-1088 or****www.fda.gov/medwatch.
Manufactured by:
Emcure Pharmaceuticals Ltd.,
Sanand, Ahmedabad – 382110, India.
Manufactured for:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

Revised: 03/2023
