Ribavirin
These highlights do not include all the information needed to use RIBAVIRIN TABLETS safely and effectively. See full prescribing information for RIBAVIRIN TABLETS. RIBAVIRIN tablets, for oral use Initial U.S. Approval: 2002
eee304d0-c2ea-44f4-97d9-92a414d31b6c
HUMAN PRESCRIPTION DRUG LABEL
Feb 6, 2024
Aurobindo Pharma Limited
DUNS: 650082092
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ribavirin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
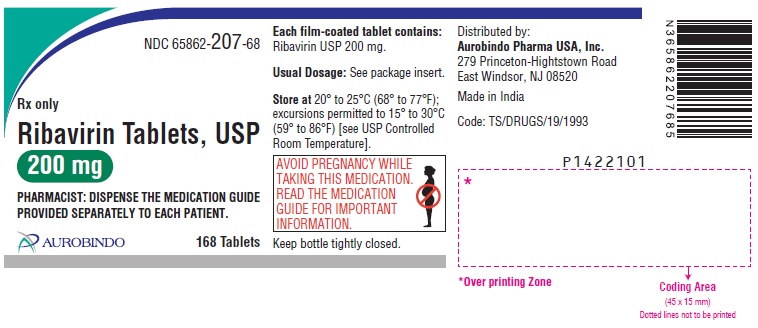
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 200 mg (168 Tablet Bottle)
NDC 65862-207-68
Rx only
Ribavirin Tablets, USP
200 mg
PHARMACIST: DISPENSE THE MEDICATION GUIDE
** PROVIDED SEPARATELY TO EACH PATIENT.**
****AUROBINDO 168 Tablets
Boxed Warning Section
WARNING: RISK OF SERIOUS DISORDERS AND RIBAVIRIN-ASSOCIATED EFFECTS
Indications & Usage Section
1 INDICATIONS AND USAGE
Ribavirin tablets in combination with PEGASYS® (peginterferon alfa-2a) are indicated for the treatment of patients 5 years of age and older with chronic hepatitis C (CHC) virus infection who have compensated liver disease and have not been previously treated with interferon alpha.
The following points should be considered when initiating ribavirin tablets combination therapy with PEGASYS®:
- This indication is based on clinical trials of combination therapy in patients with CHC and compensated liver disease, some of whom had histological evidence of cirrhosis (Child-Pugh class A), and in adult patients with clinically stable HIV disease and CD4 count greater than 100 cells/mm3.
- This indication is based on achieving undetectable HCV RNA after treatment for 24 or 48 weeks, based on HCV genotype, and maintaining a Sustained Virologic Response (SVR) 24 weeks after the last dose.
- Safety and efficacy data are not available for treatment longer than 48 weeks.
- The safety and efficacy of ribavirin tablets and PEGASYS® therapy have not been established in liver or other organ transplant recipients, patients with decompensated liver disease, or previous non-responders to interferon therapy.
- The safety and efficacy of ribavirin tablets therapy for the treatment of adenovirus, RSV, parainfluenza or influenza infections have not been established. Ribavirin tablets should not be used for these indications. Ribavirin for inhalation has a separate package insert, which should be consulted if ribavirin inhalation therapy is being considered.
Ribavirin tablets are a nucleoside analogue indicated for the treatment of chronic hepatitis C (CHC) virus infection in combination with PEGASYS® in patients 5 years of age and older with compensated liver disease not previously treated with interferon alpha, and in adult CHC patients coinfected with HIV (1)
Contraindications Section
4 CONTRAINDICATIONS
Ribavirin tablets are contraindicated in:
- Women who are pregnant. Ribavirin tablets may cause fetal harm when administered to a pregnant woman. Ribavirin tablets are contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus [see Warnings and Precautions (5.1), Use in Specific Populations (8.1), and Patient Counseling Information (17)].
- Men whose female partners are pregnant.
- Patients with hemoglobinopathies (e.g., thalassemia major or sickle-cell anemia).
- In combination with didanosine. Reports of fatal hepatic failure, as well as peripheral neuropathy, pancreatitis, and symptomatic hyperlactatemia/lactic acidosis have been reported in clinical trials [see Drug Interactions (7.1)].
Ribavirin tablets and PEGASYS® combination therapy are contraindicated in patients with:
- Autoimmune hepatitis.
- Hepatic decompensation (Child-Pugh score greater than 6; class B and C) in cirrhotic CHC monoinfected patients before treatment [see Warnings and Precautions (5.3)].
- Hepatic decompensation (Child-Pugh score greater than or equal to 6) in cirrhotic CHC patients coinfected with HIV before treatment [see Warnings and Precautions (5.3)].
- Pregnant women and men whose female partners are pregnant (4, 5.1, 8.1)
- Hemoglobinopathies (4)
- Coadministration with didanosine (4, 7.1)
Ribavirin tablets in combination with PEGASYS® are contraindicated in patients with:
- Autoimmune hepatitis (4)
- Hepatic decompensation in cirrhotic patients (4, 5.3)
Adverse Reactions Section
6 ADVERSE REACTIONS
PEGASYS® in combination with ribavirin causes a broad variety of serious adverse reactions [see Boxed Warning and Warnings and Precautions (5)]. The most common serious or life-threatening adverse reactions induced or aggravated by ribavirin/PEGASYS® include depression, suicide, relapse of drug abuse/overdose, and bacterial infections each occurring at a frequency of less than 1%. Hepatic decompensation occurred in 2% (10/574) CHC/HIV patients [see Warnings and Precautions (5.3)].
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adult Patients
In the pivotal registration trials NV15801 and NV15942, 886 patients received ribavirin for 48 weeks at doses of 1000/1200 mg based on body weight. In these trials, one or more serious adverse reactions occurred in 10% of CHC monoinfected subjects and in 19% of CHC/HIV subjects receiving PEGASYS® alone or in combination with ribavirin. The most common serious adverse event (3% in CHC and 5% in CHC/HIV) was bacterial infection (e.g., sepsis, osteomyelitis, endocarditis, pyelonephritis, pneumonia).
Other serious adverse reactions occurred at a frequency of less than 1% and included: suicide, suicidal ideation, psychosis, aggression, anxiety, drug abuse and drug overdose, angina, hepatic dysfunction, fatty liver, cholangitis, arrhythmia, diabetes mellitus, autoimmune phenomena (e.g., hyperthyroidism, hypothyroidism, sarcoidosis, systemic lupus erythematosus, rheumatoid arthritis), peripheral neuropathy, aplastic anemia, peptic ulcer, gastrointestinal bleeding, pancreatitis, colitis, corneal ulcer, pulmonary embolism, coma, myositis, cerebral hemorrhage, thrombotic thrombocytopenic purpura, psychotic disorder, and hallucination.
The percentage of patients in clinical trials who experienced one or more adverse events was 98%. The most commonly reported adverse reactions were psychiatric reactions, including depression, insomnia, irritability, anxiety, and flu-like symptoms such as fatigue, pyrexia, myalgia, headache and rigors. Other common reactions were anorexia, nausea and vomiting, diarrhea, arthralgias, injection site reactions, alopecia, and pruritus.Table 5 shows rates of adverse events occurring in greater than or equal to 5% of subjects receiving pegylated interferon and ribavirin combination therapy in the CHC Clinical Trial, NV15801.
Ten percent of CHC monoinfected patients receiving 48 weeks of therapy with PEGASYS® in combination with ribavirin discontinued therapy; 16% of CHC/HIV coinfected patients discontinued therapy. The most common reasons for discontinuation of therapy were psychiatric, flu-like syndrome (e.g., lethargy, fatigue, headache), dermatologic and gastrointestinal disorders, and laboratory abnormalities (thrombocytopenia, neutropenia, and anemia).
Overall 39% of patients with CHC or CHC/HIV required modification of PEGASYS® and/or ribavirin therapy. The most common reason for dose modification of PEGASYS® in CHC and CHC/HIV patients was for laboratory abnormalities; neutropenia (20% and 27%, respectively) and thrombocytopenia (4% and 6%, respectively). The most common reason for dose modification of ribavirin in CHC and CHC/HIV patients was anemia (22% and 16%, respectively).
PEGASYS® dose was reduced in 12% of patients receiving 1000 mg to 1200 mg ribavirin for 48 weeks and in 7% of patients receiving 800 mg ribavirin for 24 weeks. Ribavirin dose was reduced in 21% of patients receiving 1000 mg to 1200 mg ribavirin for 48 weeks and in 12% of patients receiving 800 mg ribavirin for 24 weeks.
Chronic hepatitis C monoinfected patients treated for 24 weeks with PEGASYS® and 800 mg ribavirin were observed to have lower incidence of serious adverse events (3% vs. 10%), hemoglobin less than 10 g/dL (3% vs. 15%), dose modification of PEGASYS®(30% vs. 36%) and ribavirin (19% vs. 38%), and of withdrawal from treatment (5% vs. 15%) compared to patients treated for 48 weeks with PEGASYS® and 1000 mg or 1200 mg ribavirin. On the other hand, the overall incidence of adverse events appeared to be similar in the two treatment groups.****
Table 5 Adverse Reactions Occurring in Greater Than or Equal to 5% of Patients in Chronic Hepatitis C Clinical Trials (Study NV15801)
| ||
|
Body System |
CHC Combination Therapy Study NV15801 | |
|
PEGASYS**®180 mcg + 1000 mg or 1200 mg Ribavirin Tablets48 weeks** |
Interferon alfa-2b**+ 1000 mg or** | |
|
N=451 |
N=443 | |
|
% |
% | |
|
Application Site Disorders |
23 |
16 |
|
Endocrine Disorders |
4 |
5 |
|
Flu-like Symptoms and Signs |
65 |
68 |
|
Gastrointestinal |
25 |
29 |
|
Hematologic* |
14 |
12 |
|
Metabolic and Nutritional |
24 |
26 |
|
Musculoskeletal, Connective Tissue and Bone |
40 |
49 |
|
Neurological |
43 |
49 |
|
Psychiatric |
33 |
38 |
|
Resistance Mechanism Disorders |
12 |
10 |
|
Respiratory, Thoracic and Mediastinal |
13 |
14 |
|
Skin and Subcutaneous Tissue |
28 |
33 |
|
Visual Disorders |
5 |
2 |
Pediatric Patients
In a clinical trial with 114 pediatric subjects (5 to 17 years of age) treated with PEGASYS® alone or in combination with ribavirin, dose modifications were required in approximately one-third of subjects, most commonly for neutropenia and anemia. In general, the safety profile observed in pediatric subjects was similar to that seen in adults. In the pediatric study, the most common adverse events in subjects treated with combination therapy PEGASYS® and ribavirin for up to 48 weeks were influenza-like illness (91%), upper respiratory tract infection (60%), headache (64%), gastrointestinal disorder (56%), skin disorder (47%), and injection-site reaction (45%). Seven subjects receiving combination PEGASYS® and ribavirin treatment for 48 weeks discontinued therapy for safety reasons (depression, psychiatric evaluation abnormal, transient blindness, retinal exudates, hyperglycemia, type 1 diabetes mellitus, and anemia). Severe adverse events were reported in 2 subjects in the PEGASYS® plus ribavirin combination therapy group (hyperglycemia and cholecystectomy).
Table 6 Percentage of Pediatric Subjects with Adverse Reactions* During First 24 Weeks of Treatment by Treatment Group and for 24 Weeks Post- treatment (in at Least 10% of Subjects)
| ||
|
Study NV17424 | ||
|
System Organ Class |
PEGASYS**®** |
PEGASYS**®****** |
|
% |
% | |
|
General disorders and administration site conditions | ||
|
Influenza like illness |
91 |
81 |
|
Injection site reaction |
44 |
42 |
|
Fatigue |
25 |
20 |
|
Irritability |
24 |
14 |
|
Gastrointestinal disorders | ||
|
Gastrointestinal disorder |
49 |
44 |
|
Nervous system disorders | ||
|
Headache |
51 |
39 |
|
Skin and subcutaneous tissue disorders | ||
|
Rash |
15 |
10 |
|
Pruritus |
11 |
12 |
|
Musculoskeletal, connective tissue and bone disorders | ||
|
Musculoskeletal pain |
35 |
29 |
|
Psychiatric disorders | ||
|
Insomnia |
9 |
12 |
|
Metabolism and nutrition disorders | ||
|
Decreased appetite |
11 |
14 |
In pediatric subjects randomized to combination therapy, the incidence of most adverse reactions was similar for the entire treatment period (up to 48 weeks plus 24 weeks follow-up) in comparison to the first 24 weeks, and increased only slightly for headache, gastrointestinal disorder, irritability and rash. The majority of adverse reactions occurred in the first 24 weeks of treatment.
Growth Inhibition in Pediatric Subjects [see Warnings and Precautions (5.8)].
Pediatric subjects treated with PEGASYS® plus ribavirin combination therapy showed a delay in weight and height increases with up to 48 weeks of therapy compared with baseline. Both weight for age and height for age z-scores as well as the percentiles of the normative population for subject weight and height decreased during treatment. At the end of 2 years follow-up after treatment, most subjects had returned to baseline normative curve percentiles for weight (64th mean percentile at baseline, 60 th mean percentile at 2 years post-treatment) and height (54th mean percentile at baseline, 56 th mean percentile at 2 years post-treatment). At the end of treatment, 43% (23 of 53) of subjects experienced a weight percentile decrease of more than 15 percentiles, and 25% (13 of 53) experienced a height percentile decrease of more than 15 percentiles on the normative growth curves. At 2 years post- treatment, 16% (6 of 38) of subjects were more than 15 percentiles below their baseline weight curve and 11% (4 of 38) were more than 15 percentiles below their baseline height curve.
Thirty-eight of the 114 subjects enrolled in the long-term follow-up study, extending up to 6 years post-treatment. For most subjects, post-treatment recovery in growth at 2 years post-treatment was maintained to 6 years post- treatment.
** Common Adverse Reactions in CHC with HIV Coinfection (Adults)**
The adverse event profile of coinfected patients treated with
PEGASYS®/ribavirin in Study NR15961 was generally similar to that shown for
monoinfected patients in Study NV15801 (Table 5). Events occurring more
frequently in coinfected patients were neutropenia (40%), anemia (14%),
thrombocytopenia (8%), weight decrease (16%), and mood alteration (9%).
Laboratory Test Abnormalities
Adult Patients
Anemia due to hemolysis is the most significant toxicity of ribavirin therapy. Anemia (hemoglobin less than 10 g/dL) was observed in 13% of all ribavirin and PEGASYS® combination-treated patients in clinical trials. The maximum drop in hemoglobin occurred during the first 8 weeks of initiation of ribavirin therapy [see Dosage and Administration (2.3)].
Table 7 Selected Laboratory Abnormalities During Treatment with Ribavirin in Combination With Either PEGASYS® or Interferon alfa-2b|
Laboratory Parameter |
PEGASYS**®****+ Ribavirin 1000/1200 mg 48 wks** |
Interferon alfa-2b** + Ribavirin 1000/1200 mg** |
|
(N=887) |
(N=443) | |
|
Neutrophils (cells/mm3) | ||
|
1,000 <1,500 |
34% |
38% |
|
500 <1,000 |
49% |
21% |
|
<500 |
5% |
1% |
|
Platelets (cells/mm3) | ||
|
50,000 to <75,000 |
11% |
4% |
|
20,000 to <50,000 |
5% |
< 1% |
|
<20,000 |
0 |
0 |
|
Hemoglobin (g/dL) | ||
|
8.5 to 9.9 |
11% |
11% |
|
<8.5 |
2% |
< 1% |
Pediatric Patients
Decreases in hemoglobin, neutrophils and platelets may require dose reduction or permanent discontinuation from treatment [see Dosage and Administration (2.4)]. Most laboratory abnormalities noted during the clinical trial returned to baseline levels shortly after discontinuation of treatment.
Table 8 Selected Hematologic Abnormalities During First 24 Weeks of Treatment by Treatment Group in Previously Untreated Pediatric Subjects
| ||
|
Laboratory Parameter |
PEGASYS**®** |
PEGASYS**®** |
|
Neutrophils (cells/mm3) | ||
|
1,000 to <1,500 |
31% |
39% |
|
750 to <1,000 |
27% |
17% |
|
500 to <750 |
25% |
15% |
|
<500 |
7% |
5% |
|
Platelets (cells/mm3) | ||
|
75,000 to <100,000 |
4% |
2% |
|
50,000 to <75,000 |
0% |
2% |
|
<50,000 |
0% |
0% |
|
Hemoglobin (g/dL) | ||
|
8.5 to <10 |
7% |
3% |
|
<8.5 |
0% |
0% |
In patients randomized to combination therapy, the incidence of abnormalities during the entire treatment phase (up to 48 weeks plus 24 weeks follow-up) in comparison to the first 24 weeks increased slightly for neutrophils between 500 and 1,000 cells/mm³ and hemoglobin values between 8.5 and 10 g/dL. The majority of hematologic abnormalities occurred in the first 24 weeks of treatment.
6.2 Postmarketing Experience
The following adverse reactions have been identified and reported during post- approval use of PEGASYS®/ribavirin combination therapy. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System disorders
Pure red cell aplasia
Ear and Labyrinth disorders
Hearing impairment, hearing loss
Eye disorders
Serous retinal detachment
Immune disorders
Liver and renal graft rejection
Metabolism and Nutrition disorders
Dehydration
Skin and Subcutaneous Tissue disorders
Stevens-Johnson syndrome (SJS)
Toxic epidermal necrolysis (TEN)
The most common adverse reactions (frequency greater than 40%) in adults receiving combination therapy are fatigue/asthenia, pyrexia, myalgia, and headache. (6.1)
The most common adverse reactions in pediatric subjects were similar to those seen in adults. (6.1)
** To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.**
Drug Interactions Section
7 DRUG INTERACTIONS
Results from a pharmacokinetic sub-study demonstrated no pharmacokinetic interaction between PEGASYS® (peginterferon alfa-2a) and ribavirin.
7.1 Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
In vitro data indicate ribavirin reduces phosphorylation of lamivudine, stavudine, and zidovudine. However, no pharmacokinetic (e.g., plasma concentrations or intracellular triphosphorylated active metabolite concentrations) or pharmacodynamic (e.g., loss of HIV/HCV virologic suppression) interaction was observed when ribavirin and lamivudine (n=18), stavudine (n=10), or zidovudine (n=6) were coadministered as part of a multi- drug regimen to HCV/HIV coinfected patients.
In Study NR15961 among the CHC/HIV coinfected cirrhotic patients receiving NRTIs, cases of hepatic decompensation (some fatal) were observed [see Warnings and Precautions (5.3)].
Patients receiving PEGASYS®/ribavirin and NRTIs should be closely monitored for treatment-associated toxicities. Physicians should refer to prescribing information for the respective NRTIs for guidance regarding toxicity management. In addition, dose reduction or discontinuation of PEGASYS®, ribavirin or both should also be considered if worsening toxicities are observed, including hepatic decompensation (e.g., Child-Pugh greater than or equal to 6) [see Warnings and Precautions (5.3) and Dosage and Administration (2.3)].
Didanosine
Coadministration of ribavirin and didanosine is contraindicated. Didanosine or its active metabolite (dideoxyadenosine 5’-triphosphate) concentrations are increased when didanosine is coadministered with ribavirin, which could cause or worsen clinical toxicities. Reports of fatal hepatic failure as well as peripheral neuropathy, pancreatitis, and symptomatic hyperlactatemia/lactic acidosis have been reported in clinical trials [see Contraindications (4)].
Zidovudine
In Study NR15961, patients who were administered zidovudine in combination with PEGASYS®/ribavirin developed severe neutropenia (ANC less than 500) and severe anemia (hemoglobin less than 8 g/dL) more frequently than similar patients not receiving zidovudine (neutropenia 15% vs. 9%) (anemia 5% vs. 1%). Discontinuation of zidovudine should be considered as medically appropriate.
7.2 Drugs Metabolized by Cytochrome P450
In vitro studies indicate that ribavirin does not inhibit CYP 2C9, CYP 2C19, CYP 2D6 or CYP 3A4.
7.3 Azathioprine
The use of ribavirin to treat chronic hepatitis C in patients receiving azathioprine has been reported to induce severe pancytopenia and may increase the risk of azathioprine-related myelotoxicity. Inosine monophosphate dehydrogenase (IMDH) is required for one of the metabolic pathways of azathioprine. Ribavirin is known to inhibit IMDH, thereby leading to accumulation of an azathioprine metabolite, 6-methylthioinosine monophosphate (6-MTITP), which is associated with myelotoxicity (neutropenia, thrombocytopenia, and anemia). Patients receiving azathioprine with ribavirin should have complete blood counts, including platelet counts, monitored weekly for the first month, twice monthly for the second and third months of treatment, then monthly or more frequently if dosage or other therapy changes are necessary [see Warnings and Precautions (5.6)].
- Nucleoside analogues: Closely monitor for toxicities. Discontinue nucleoside reverse transcriptase inhibitors or reduce dose or discontinue interferon, ribavirin or both with worsening toxicities (7.1)
- Azathioprine: Concomitant use of azathioprine with ribavirin has been reported to induce severe pancytopenia and may increase the risk of azathioprine-related myelotoxicity (7.3)
Dosage & Administration Section
2 DOSAGE AND ADMINISTRATION
Ribavirin tablets should be taken with food. Ribavirin tablets should be given in combination with PEGASYS®; it is important to note that ribavirin tablets should never be given as monotherapy. See PEGASYS® Package Insert for all instructions regarding PEGASYS® dosing and administration.
2.1 Chronic Hepatitis C Monoinfection
Adult Patients
The recommended dose of ribavirin tablets is provided inTable 1. The recommended duration of treatment for patients previously untreated with ribavirin and interferon is 24 to 48 weeks.
The daily dose of ribavirin tablets is 800 mg to 1200 mg administered orally in two divided doses. The dose should be individualized to the patient depending on baseline disease characteristics (e.g., genotype), response to therapy, and tolerability of the regimen (seeTable 1).
Table 1 PEGASYS® and Ribavirin Tablets Dosing Recommendations|
Genotypes 2 and 3 showed no increased response to treatment beyond 24 weeks
(seeTable 10).
| |||
|
Hepatitis C Virus |
PEGASYS**®**** Dose*** |
Ribavirin****Tablets |
Duration |
|
Genotypes 1, 4 |
180 mcg |
<75 kg = 1000 mg |
48 weeks |
|
Genotypes 2, 3 |
180 mcg |
800 mg |
24 weeks |
Pediatric Patients
PEGASYS® is administered as 180 mcg/1.73m2 x BSA once weekly subcutaneously, to a maximum dose of 180 mcg, and should be given in combination with ribavirin. The recommended treatment duration for patients with genotype 2 or 3 is 24 weeks and for other genotypes is 48 weeks.
Ribavirin tablets should be given in combination with PEGASYS®. Ribavirin tablets are available only as a 200 mg tablet and therefore the healthcare provider should determine if this sized tablet can be swallowed by the pediatric patient. The recommended doses for ribavirin tablets are provided in Table 2. Patients who initiate treatment prior to their 18th birthday should maintain pediatric dosing through the completion of therapy.
Table 2 Ribavirin Tablets Dosing Recommendations for Pediatric Patients|
*approximately 15 mg/kg/day | ||
|
Body Weight in kilograms (kg) |
Ribavirin Tablets |
Ribavirin Tablets |
|
23 to 33 |
400 mg/day |
1 x 200 mg tablet A.M. |
|
34 to 46 |
600 mg/day |
1 x 200 mg tablet A.M. |
|
47 to 59 |
800 mg/day |
2 x 200 mg tablets A.M. |
|
60 to 74 |
1000 mg/day |
2 x 200 mg tablets A.M. |
|
≥75 |
1200 mg/day |
3 x 200 mg tablets A.M. |
2.2 Chronic Hepatitis C with HIV Coinfection
Adult Patients
The recommended dose for treatment of chronic hepatitis C in patients coinfected with HIV is PEGASYS® 180 mcg subcutaneous once weekly and ribavirin tablets 800 mg by mouth daily for a total duration of 48 weeks, regardless of HCV genotype.
2.3 Dose Modifications
Adult and Pediatric Patients
If severe adverse reactions or laboratory abnormalities develop during combination ribavirin tablets/PEGASYS® therapy, the dose should be modified or discontinued, if appropriate, until the adverse reactions abate or decrease in severity. If intolerance persists after dose adjustment, ribavirin tablets/PEGASYS® therapy should be discontinued.Table 3 provides guidelines for dose modifications and discontinuation based on the patient’s hemoglobin concentration and cardiac status.
Ribavirin tablets should be administered with caution to patients with pre- existing cardiac disease. Patients should be assessed before commencement of therapy and should be appropriately monitored during therapy. If there is any deterioration of cardiovascular status, therapy should be stopped [see Warnings and Precautions (5.2)].
Table 3 Ribavirin Tablets Dose Modification Guidelines in Adults and Pediatrics|
Body weight in kilograms (kg) |
Laboratory Values | |
|
Hemoglobin <10 g/dL in patients with no cardiac disease, or Decrease in hemoglobin of ≥2 g/dL during any 4 week period in patients with history of stable cardiac disease |
Hemoglobin <8.5 g/dL in patients with no cardiac disease, or Hemoglobin <12 g/dL despite 4 weeks at reduced dose in patients with history of stable cardiac disease | |
|
Adult Patients older than 18 years of age | ||
|
Any weight |
1 x 200 mg tablet A.M. |
Discontinue ribavirin tablets |
|
Pediatric Patients 5 to 18 years of age | ||
|
23 to 33 kg |
1 x 200 mg tablet A.M. |
Discontinue ribavirin tablets |
|
34 to 46 kg |
1 x 200 mg tablet A.M. | |
|
47 to 59 kg |
1 x 200 mg tablet A.M. | |
|
60 to 74 kg |
1 x 200 mg tablet A.M. | |
|
≥75 kg |
1 x 200 mg tablet A.M. |
The guidelines for ribavirin tablets dose modifications outlined in this table also apply to laboratory abnormalities or adverse reactions other than decreases in hemoglobin values.
Adult Patients
Once ribavirin tablets have been withheld due to either a laboratory abnormality or clinical adverse reaction, an attempt may be made to restart ribavirin tablets at 600 mg daily and further increase the dose to 800 mg daily. However, it is not recommended that ribavirin tablets be increased to the original assigned dose (1000 mg to 1200 mg).
Pediatric Patients
Upon resolution of a laboratory abnormality or clinical adverse reaction, an increase in ribavirin tablets dose to the original dose may be attempted depending upon the physician’s judgment. If ribavirin tablets have been withheld due to a laboratory abnormality or clinical adverse reaction, an attempt may be made to restart ribavirin tablets at one-half the full dose.
2.4 Renal Impairment
The total daily dose of ribavirin tablets should be reduced for patients with creatinine clearance less than or equal to 50 mL/min; and the weekly dose of PEGASYS® should be reduced for creatinine clearance less than 30 mL/min as follows inTable 4[see Use in Specific Populations (8.7), Pharmacokinetics (12.3), and PEGASYS® Package Insert].
Table 4 Dosage Modification for Renal Impairment|
Creatinine Clearance |
PEGASYS**®**** Dose** |
Ribavirin Tablets Dose (daily) |
|
30 to 50 mL/min |
180 mcg |
Alternating doses, 200 mg and |
|
Less than 30 mL/min |
135 mcg |
200 mg daily |
|
Hemodialysis |
135 mcg |
200 mg daily |
The dose of ribavirin tablets should not be further modified in patients with renal impairment. If severe adverse reactions or laboratory abnormalities develop, ribavirin tablets should be discontinued, if appropriate, until the adverse reactions abate or decrease in severity. If intolerance persists after restarting ribavirin tablets, ribavirin tablets/PEGASYS® therapy should be discontinued.
No data are available for pediatric subjects with renal impairment.
2.5 Discontinuation of Dosing
Discontinuation of PEGASYS®/ribavirin tablets therapy should be considered if the patient has failed to demonstrate at least a 2 log10 reduction from baseline in HCV RNA by 12 weeks of therapy, or undetectable HCV RNA levels after 24 weeks of therapy.
PEGASYS®/Ribavirin tablets therapy should be discontinued in patients who develop hepatic decompensation during treatment [see Warnings and Precautions (5.3)].
- CHC: Ribavirin tablets are administered according to body weight and genotype (2.1)
- CHC with HIV coinfection: 800 mg by mouth daily for a total of 48 weeks, regardless of genotype (2.2)
- Dose reduction or discontinuation is recommended in patients experiencing certain adverse reactions or renal impairment (2.3, 2.4)
Dosage Forms & Strengths Section
3 DOSAGE FORMS AND STRENGTHS
Ribavirin is available as a light pink colored, capsule shaped, film-coated tablet for oral administration. Each tablet contains 200 mg of ribavirin.
- Ribavirin tablets, USP 200 mg (3)
Overdosage Section
10 OVERDOSAGE
No cases of overdose with ribavirin have been reported in clinical trials. Hypocalcemia and hypomagnesemia have been observed in persons administered greater than the recommended dosage of ribavirin. In most of these cases, ribavirin was administered intravenously at dosages up to and in some cases exceeding four times the recommended maximum oral daily dose.
Nonclinical Toxicology Section
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a p53 (+/-) mouse carcinogenicity study up to the maximum tolerated dose of 100 mg/kg/day, ribavirin was not oncogenic. Ribavirin was also not oncogenic in a rat 2-year carcinogenicity study at doses up to the maximum tolerated dose of 60 mg/kg/day. On a body surface area basis, these doses are approximately 0.5 and 0.6 times the maximum recommended daily human dose of ribavirin, respectively.****
** Mutagenesis**
****Ribavirin demonstrated mutagenic activity in the in vitro mouse lymphoma assay. No clastogenic activity was observed in an in vivo mouse micronucleus assay at doses up to 2000 mg/kg. However, results from studies published in the literature show clastogenic activity in the in vivo mouse micronucleus assay at oral doses up to 2000 mg/kg. A dominant lethal assay in rats was negative, indicating that if mutations occurred in rats they were not transmitted through male gametes.
** Impairment of Fertility**
****In a fertility study in rats, ribavirin showed a marginal reduction in sperm counts at the dose of 100 mg/kg/day with no effect on fertility. Upon cessation of treatment, total recovery occurred after 1 spermatogenesis cycle. Abnormalities in sperm were observed in studies in mice designed to evaluate the time course and reversibility of ribavirin-induced testicular degeneration at doses of 15 to 150 mg/kg/day (approximately 0.1 to 0.8 times the maximum recommended daily human dose of ribavirin) administered for 3 to 6 months. Upon cessation of treatment, essentially total recovery from ribavirin-induced testicular toxicity was apparent within 1 or 2 spermatogenic cycles.
Female patients of childbearing potential and male patients with female partners of childbearing potential should not receive ribavirin unless the patient and his/her partner are using effective contraception (two reliable forms). Based on a multiple dose half-life (t1/2) of ribavirin of 12 days, effective contraception must be utilized for 6 months post therapy (i.e., 15 half-lives of clearance for ribavirin).
No reproductive toxicology studies have been performed using PEGASYS® in combination with ribavirin. However, peginterferon alfa-2a and ribavirin when administered separately, each has adverse effects on reproduction. It should be assumed that the effects produced by either agent alone would also be caused by the combination of the two agents.
13.2 Animal Toxicology
In a study in rats, it was concluded that dominant lethality was not induced by ribavirin at doses up to 200 mg/kg for 5 days (up to 1.7 times the maximum recommended human dose of ribavirin).
Long-term studies in the mouse and rat (18 to 24 months; dose 20 to 75, and 10 to 40 mg/kg/day, respectively, approximately 0.1 to 0.4 times the maximum daily human dose of ribavirin) have demonstrated a relationship between chronic ribavirin exposure and an increased incidence of vascular lesions (microscopic hemorrhages) in mice. In rats, retinal degeneration occurred in controls, but the incidence was increased in ribavirin-treated rats.
How Supplied Section
16 HOW SUPPLIED/STORAGE AND HANDLING
Ribavirin Tablets USP, 200 mg are light pink colored, capsule shaped, film-coated tablets debossed with ‘F’ on one side and ‘10’ on the other side.
Bottles of 168 NDC 65862-207-68
Bottles of 500 NDC 65862-207-05
Storage and Handling
Store at20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep bottle tightly closed.
SPL MEDGUIDE SECTION
MEDICATION GUIDE
Ribavirin Tablets, USP
****(rye" ba vye' rin)
Read this Medication Guide carefully before you start taking ribavirin tablets and read the Medication Guide each time you get more ribavirin tablets. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Also read the Medication Guide for PEGASYS® (peginterferon alfa-2a).
What is the most important information I should know about ribavirin tablets?
**1. You should not take ribavirin tablets alone to treat chronic hepatitis C infection.**Ribavirin tablets should be used with PEGASYS® to treat chronic hepatitis C infection.
** 2. Ribavirin tablets may cause you to have a blood problem (hemolytic anemia) that can worsen any heart problems you have, and cause you to have a heart attack or die.** Tell your healthcare provider if you have ever had any heart problems. Ribavirin tablets may not be right for you. If you have chest pain while you take ribavirin tablets, get emergency medical attention right away.
3. Ribavirin tablets may cause birth defects or death of your unborn baby. If you are pregnant or your sexual partner is pregnant, do not take ribavirin tablets. You or your sexual partner should not become pregnant while you take ribavirin tablets and for 6 months after treatment is over. You must use two forms of birth control when you take ribavirin tablets and for the 6 months after treatment.
- Females must have a pregnancy test before starting ribavirin tablets, every month while treated with ribavirin tablets, and every month for the 6 months after treatment with ribavirin tablets. *If you or your female sexual partner becomes pregnant while taking ribavirin tablets or within 6 months after you stop taking ribavirin tablets, tell your healthcare provider right away.
What are ribavirin tablets?
Ribavirin tablets are a prescription medicine used with another medicine called PEGASYS® (peginterferon alfa-2a) to treat chronic (lasting a long time) hepatitis C infection in people 5 years and older whose liver still works normally, and who have not been treated before with a medicine called an interferon alpha. It is not known if ribavirin tablets are safe and will work in children under 5 years of age.
** Who should not take ribavirin tablets?**
** See “What is the most important information I should know about ribavirin tablets?”**
Do not take ribavirin tablets if you:
*have certain types of hepatitiscaused by your immune system attacking your liver (autoimmune hepatitis) *have certain blood disorders, such as thalassemia major or sickle-cell anemia (hemoglobinopathies) *take didanosine (Videx or Videx EC)
Talk to your healthcare provider before starting treatment with ribavirin tablets if you have any of these medical conditions.
** What should I tell my healthcare provider before taking ribavirin tablets?**
****Before you take ribavirin tablets, tell your healthcare provider if you have or have had:
*treatment for hepatitis C that did not work for you *serious allergic reactions to ribavirin tablets or to any of the ingredients in ribavirin tablets. See the end of this Medication Guide for a list of ingredients. ***breathing problems.**Ribavirin tablets may cause or worsen your breathing problems you already have. *vision problems. Ribavirin tablets may cause eye problems or worsen eye problems you already have. You should have an eye exam before you start treatment with ribavirin tablets. *certain blood disorders such as anemia *high blood pressure, heart problems or have had a heart attack. Your healthcare provider should test your blood and heart before you start treatment with ribavirin tablets. *thyroid problems ***diabetes.**Ribavirin tablets and PEGASYS® combination therapy may make your diabetes worse or harder to treat. *liver problems other than hepatitis C virus infection *human immunodeficiency virus (HIV) or other immunity problems ***mental health problems,**including depression or thoughts of suicide *kidney problems *an organ transplant *drug addiction or abuse *infection with hepatitis B virus *any other medical condition *are breast feeding. It is not known if ribavirin passes into your breast milk. You and your healthcare provider should decide if you will take ribavirin tablets or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Some medicines can cause serious side effects if taken while you also take ribavirin tablets. Some medicines may affect how ribavirin tablets work or ribavirin tablets may affect how your other medicines work.
Especially tell your healthcare provider if you take any medicines to treat HIV, including didanosine (Videx or Videx EC), or if you take azathioprine (Imuran or Azasan).
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take ribavirin tablets?
- Take ribavirin tablets exactly as your healthcare provider tells you. Your healthcare provider will tell you how much ribavirin tablets to take and when to take them. For children 5 years of age and older your healthcare provider will prescribe the dose of ribavirin tablets based on weight.
- Take ribavirin tablets with food.
- If you miss a dose of ribavirin tablets, take the missed dose as soon as possible during the same day. Do not double the next dose. If you have questions about what to do, call your healthcare provider.
- If you take too much ribavirin tablets, call your healthcare provider or local Poison Control Center right away, or go the nearest hospital emergency room right away.
- Your healthcare provider should do blood tests before you start treatment with ribavirin tablets, at weeks 2 and 4 of treatment, and then as needed to see how well you are tolerating treatment and to check for side effects. Your healthcare provider may change your dose of ribavirin tablets based on blood test results or side effects you may have.
- If you have heart problems, your healthcare provider should check your heart by doing an electrocardiogram before you start treatment with ribavirin tablets, and if needed during treatment.
What should I avoid while taking ribavirin tablets?
*Ribavirin tablets can make you feel tired, dizzy, or confused. You should not drive or operate machinery if you have any of these symptoms. *Do not drink alcohol, including beer, wine, and liquor. This may make your liver disease worse.
What are the possible side effects of ribavirin tablets?
Ribavirin tablets may cause serious side effects including:
See “What is the most important information I should know about ribavirin tablets?”
*Swelling and irritation of your pancreas (pancreatitis). You may have stomach pain, nausea, vomiting or diarrhea. *Severe allergic reactions. Symptoms may include hives, wheezing, trouble breathing, chest pain, swelling of your mouth, tongue, or lips, or severe rash. *Serious breathing problems. Difficulty breathing may be a sign of a serious lung infection (pneumonia) that can lead to death. *Serious eye problemsthat may lead to vision loss or blindness. *Liver problems. Some people may get worsening of liver function. Tell your healthcare provider right away if you have any of these symptoms: stomach bloating, confusion, brown urine, and yellow eyes. *Severe depression *Suicidal thoughts and attempts *Effect on growth in children. Children can experience a delay in weight gain and height increase while being treated with PEGASYS® and ribavirin tablets. Catch-up in growth happens after treatment stops, but some children may not reach the height that they were expected to have before treatment. Talk to your healthcare provider if you are concerned about your child’s growth during treatment with PEGASYS® and ribavirin tablets.
**** Call your healthcare provider or get medical help right away if you have any of the symptoms listed above. These may be signs of a serious side effect of ribavirin tablets treatment******.**
** Common side effects of ribavirin tablets taken with PEGASYS****®**** include:**
- flu-like symptoms-feeling tired, headache, shaking along with high temperature (fever), and muscle or joint aches
- mood changes, feeling irritable, anxiety, and difficulty sleeping
- loss of appetite, nausea, vomiting, and diarrhea
- hair loss
- itching
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of ribavirin tablets treatment. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Aurobindo Pharma USA, Inc. at 1-866-850-2876.
How should I store****ribavirin tablets?
- Store ribavirin tablets at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
- Keep the bottle tightly closed.
** Keep ribavirin tablets and all medicines out of the reach of children.**
General information about the safe and effective use of ribavirin tablets
It is not known if treatment with ribavirin tablets in combination with PEGASYS® will prevent an infected person from spreading the hepatitis C virus to another person while on treatment.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ribavirin tablets for a condition for which it was not prescribed. Do not give ribavirin tablets to other people, even if they have the same symptoms that you have. They may harm them.
This Medication Guide summarizes the most important information about ribavirin tablets. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about ribavirin tablets that is written for healthcare professionals.****
** What are the ingredients in ribavirin tablets?**
****Active Ingredient: ribavirin
Inactive Ingredients: microcrystalline cellulose, pregelatinised starch (maize), sodium starch glycolate, povidone (Kollidon 30), colloidal silicon dioxide, magnesium stearate, ethyl cellulose, triacetin, hypromellose, iron oxide red, titanium dioxide, and yellow iron oxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Trademarks are the property of their respective owners.
Distributed by:
Aurobindo Pharma USA, Inc.
****279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
****Hyderabad–500 032, India
Revised: 05/2023
Use In Specific Populations Section
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy: Category X [see Contraindications (4)].
Ribavirin produced significant embryocidal and/or teratogenic effects in all animal species in which adequate studies have been conducted. Malformations of the skull, palate, eye, jaw, limbs, skeleton, and gastrointestinal tract were noted. The incidence and severity of teratogenic effects increased with escalation of the drug dose. Survival of fetuses and offspring was reduced [see Contraindications (4) and Warnings and Precautions (5.1)].
In conventional embryotoxicity/teratogenicity studies in rats and rabbits, observed no-effect dose levels were well below those for proposed clinical use (0.3 mg/kg/day for both the rat and rabbit; approximately 0.06 times the recommended daily human dose of ribavirin). No maternal toxicity or effects on offspring were observed in a peri/postnatal toxicity study in rats dosed orally at up to 1 mg/kg/day (approximately 0.01 times the maximum recommended daily human dose of ribavirin).
** Treatment and Post-Treatment: Potential Risk to the Fetus**
Ribavirin is known to accumulate in intracellular components from where it is cleared very slowly. It is not known whether ribavirin is contained in sperm, and if so, will exert a potential teratogenic effect upon fertilization of the ova. However, because of the potential human teratogenic effects of ribavirin, male patients should be advised to take every precaution to avoid risk of pregnancy for their female partners.
Ribavirin should not be used by pregnant women or by men whose female partners are pregnant. Female patients of childbearing potential and male patients with female partners of childbearing potential should not receive ribavirin unless the patient and his/her partner are using effective contraception (two reliable forms) during therapy and for 6 months post therapy [see Contraindications (4)].
8.3 Nursing Mothers
It is not known whether ribavirin is excreted in human milk. Because many drugs are excreted in human milk and to avoid any potential for serious adverse reactions in nursing infants from ribavirin, a decision should be made either to discontinue nursing or therapy with ribavirin, based on the importance of the therapy to the mother.
8.4 Pediatric Use
Pharmacokinetic evaluations in pediatric patients have not been performed.
Safety and effectiveness of ribavirin have not been established in patients below the age of 5 years.
8.5 Geriatric Use
Clinical studies of ribavirin and PEGASYS® did not include sufficient numbers of subjects aged 65 or over to determine whether they respond differently from younger subjects. Specific pharmacokinetic evaluations for ribavirin in the elderly have not been performed. The risk of toxic reactions to this drug may be greater in patients with impaired renal function. The dose of ribavirin should be reduced in patients with creatinine clearance less than or equal to 50 mL/min; and the dose of PEGASYS® should be reduced in patients with creatinine clearance less than 30 mL/min [see Dosage and Administration (2.4); Use in Specific Populations (8.7)].
8.6 Race
A pharmacokinetic study in 42 subjects demonstrated there is no clinically significant difference in ribavirin pharmacokinetics among Black (n=14), Hispanic (n=13) and Caucasian (n=15) subjects.
8.7 Renal Impairment
Renal function should be evaluated in all patients prior to initiation of ribavirin by estimating the patient’s creatinine clearance.
A clinical trial evaluated treatment with ribavirin and PEGASYS® in 50 CHC subjects with moderate (creatinine clearance 30 to 50 mL/min) or severe (creatinine clearance less than 30 mL/min) renal impairment or end stage renal disease (ESRD) requiring chronic hemodialysis (HD). In 18 subjects with ESRD receiving chronic HD, ribavirin was administered at a dose of 200 mg daily with no apparent difference in the adverse event profile in comparison to subjects with normal renal function. Dose reductions and temporary interruptions of ribavirin (due to ribavirin-related adverse reactions, mainly anemia) were observed in up to one-third ESRD/HD subjects during treatment; and only one-third of these subjects received ribavirin for 48 weeks. Ribavirin plasma exposures were approximately 20% lower in subjects with ESRD on HD compared to subjects with normal renal function receiving the standard 1000/1200 mg ribavirin daily dose.
Subjects with moderate (n=17) or severe (n=14) renal impairment did not tolerate 600 mg or 400 mg daily doses of ribavirin, respectively, due to ribavirin-related adverse reactions, mainly anemia, and exhibited 20% to 30% higher ribavirin plasma exposures (despite frequent dose modifications) compared to subjects with normal renal function (creatinine clearance greater than 80 mL/min) receiving the standard dose of ribavirin. Discontinuation rates were higher in subjects with severe renal impairment compared to that observed in subjects with moderate renal impairment or normal renal function. Pharmacokinetic modeling and simulation indicate that a dose of 200 mg daily in patients with severe renal impairment and a dose of 200 mg daily alternating with 400 mg the following day in patients with moderate renal impairment will provide plasma ribavirin exposure similar to patients with normal renal function receiving the approved regimen of ribavirin. These doses have not been studied in patients [see Dosage and Administration (2.4), and Clinical Pharmacology (12.3)].
Based on the pharmacokinetic and safety results from this trial, patients with creatinine clearance less than or equal to 50 mL/min should receive a reduced dose of ribavirin; and patients with creatinine clearance less than 30 mL/min should receive a reduced dose of PEGASYS®. The clinical and hematologic status of patients with creatinine clearance less than or equal to 50 mL/min receiving ribavirin should be carefully monitored. Patients with clinically significant laboratory abnormalities or adverse reactions which are persistently severe or worsening should have therapy withdrawn [see Dosage and Administration (2.4), Clinical Pharmacology (12.3), and PEGASYS® Package Insert].
8.8 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of ribavirin following administration of ribavirin has not been evaluated. The clinical trials of ribavirin were restricted to patients with Child-Pugh class A disease.
8.9 Gender
No clinically significant differences in the pharmacokinetics of ribavirin were observed between male and female subjects.
Ribavirin pharmacokinetics, when corrected for weight, are similar in male and female patients.
8.10 Organ Transplant Recipients
The safety and efficacy of PEGASYS® and ribavirin treatment have not been established in patients with liver and other transplantations. As with other alpha interferons, liver and renal graft rejections have been reported on PEGASYS®, alone or in combination with ribavirin [see Adverse Reactions (6.2)].
- Pediatrics: Safety and efficacy in pediatric patients less than 5 years old have not been established (8.4)
- Renal Impairment: Dose should be reduced in patients with creatinine clearance less than or equal to 50 mL/min (8.7)
- Organ Transplant: Safety and efficacy have not been studied (8.10)
Description Section
11 DESCRIPTION
Ribavirin, is a nucleoside analogue with antiviral activity. The chemical name of ribavirin is 1-β-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide and has the following structural formula:
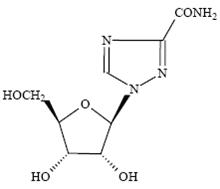
The molecular formula of ribavirin is C8H12N4O5 and the molecular weight is 244.2. Ribavirin USP is a white crystalline powder. It is freely soluble in water and slightly soluble in anhydrous alcohol.
Ribavirin USP is available as a light pink colored, capsule shaped, film- coated tablet for oral administration. Each tablet contains 200 mg of ribavirin USP and the following inactive ingredients: microcrystalline cellulose, pregelatinised starch (maize), sodium starch glycolate, povidone (Kollidon 30), colloidal silicon dioxide, magnesium stearate, ethyl cellulose, triacetin, hypromellose, iron oxide red, titanium dioxide, and yellow iron oxide.
Clinical Pharmacology Section
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ribavirin is an antiviral drug [see Microbiology (12.4)].
12.3 Pharmacokinetics
Multiple dose ribavirin pharmacokinetic data are available for HCV patients who received ribavirin in combination with peginterferon alfa-2a. Following administration of 1200 mg/day with food for 12 weeks mean±SD (n=39; body weight greater than 75 kg) AUC0-12 hr was 25,361±7110 ng·hr/mL and Cmax was 2748±818 ng/mL. The average time to reach Cmax was 2 hours. Trough ribavirin plasma concentrations following 12 weeks of dosing with food were 1662±545 ng/mL in HCV infected patients who received 800 mg/day (n=89), and 2112±810 ng/mL in patients who received 1200 mg/day (n=75; body weight greater than 75 kg).
The terminal half-life of ribavirin following administration of a single oral dose of ribavirin is about 120 to 170 hours. The total apparent clearance following administration of a single oral dose of ribavirin is about 26 L/h. There is extensive accumulation of ribavirin after multiple dosing (twice daily) such that the Cmax at steady state was four-fold higher than that of a single dose.
** Effect of Food on Absorption of Ribavirin**
Bioavailability of a single oral dose of ribavirin was increased by coadministration with a high-fat meal. The absorption was slowed (Tmax was doubled) and the AUC0-192 h and Cmax increased by 42% and 66%, respectively, when ribavirin was taken with a high-fat meal compared with fasting conditions [see Dosage and Administration (2) and Patient Counseling Information (17)].
** Elimination and Metabolism**
The contribution of renal and hepatic pathways to ribavirin elimination after administration of ribavirin is not known. In vitro studies indicate that ribavirin is not a substrate of CYP450 enzymes.
** Renal Impairment**
A clinical trial evaluated 50 CHC subjects with either moderate (creatinine clearance 30 to 50 mL/min) or severe (creatinine clearance less than 30 mL/min) renal impairment or end stage renal disease (ESRD) requiring chronic hemodialysis (HD). The apparent clearance of ribavirin was reduced in subjects with creatinine clearance less than or equal to 50 mL/min, including subjects with ESRD on HD, exhibiting approximately 30% of the value found in subjects with normal renal function. Pharmacokinetic modeling and simulation indicates that a dose of 200 mg daily in patients with severe renal impairment and a dose of 200 mg daily alternating with 400 mg the following day in patients with moderate renal impairment will provide plasma ribavirin exposures similar to that observed in patients with normal renal function receiving the standard 1000/1200 mg ribavirin daily dose. These doses have not been studied in patients.
In 18 subjects with ESRD receiving chronic HD, ribavirin was administered at a dose of 200 mg daily. Ribavirin plasma exposures in these subjects were approximately 20% lower compared to subjects with normal renal function receiving the standard 1000/1200 mg ribavirin daily dose [see Dosage and Administration (2.4), Use in Specific Populations (8.7)].
Plasma ribavirin is removed by hemodialysis with an extraction ratio of approximately 50%; however, due to the large volume of distribution of ribavirin, plasma exposure is not expected to change with hemodialysis.
12.4 Microbiology
Mechanism of Action
The mechanism by which ribavirin contributes to its antiviral efficacy in the clinic is not fully understood. Ribavirin has direct antiviral activity in tissue culture against many RNA viruses. Ribavirin increases the mutation frequency in the genomes of several RNA viruses and ribavirin triphosphate inhibits HCV polymerase in a biochemical reaction.****
** Antiviral Activity in Cell Culture**
In the stable HCV cell culture model system (HCV replicon), ribavirin inhibited autonomous HCV RNA replication with a 50% effective concentration (EC50) value of 11 to 21 mcM. In the same model, PEG-IFN α-2a also inhibited HCV RNA replication, with an EC50 value of 0.1 to 3 ng/mL. The combination of PEG-IFN α-2a and ribavirin was more effective at inhibiting HCV RNA replication than either agent alone.
** Resistance**
****Different HCV genotypes display considerable clinical variability in their response to PEG-IFN-α and ribavirin therapy. Viral genetic determinants associated with the variable response have not been definitively identified.
** Cross-resistance**
****Cross-resistance between IFN α and ribavirin has not been observed.
Clinical Studies Section
14 CLINICAL STUDIES
14.1 Chronic Hepatitis C Patients
Adult Patients
The safety and effectiveness of PEGASYS® in combination with ribavirin for the treatment of hepatitis C virus infection were assessed in two randomized controlled clinical trials. All patients were adults, had compensated liver disease, detectable hepatitis C virus, liver biopsy diagnosis of chronic hepatitis, and were previously untreated with interferon. Approximately 20% of patients in both studies had compensated cirrhosis (Child-Pugh class A). Patients coinfected with HIV were excluded from these studies.
In Study NV15801, patients were randomized to receive either PEGASYS® 180 mcg subcutaneous once weekly with an oral placebo, PEGASYS® 180 mcg once weekly with ribavirin 1000 mg by mouth (body weight less than 75 kg) or 1200 mg by mouth (body weight greater than or equal to 75 kg) or interferon alfa-2b 3 MIU subcutaneous three times a week plus ribavirin 1000 mg or 1200 mg by mouth. All patients received 48 weeks of therapy followed by 24 weeks of treatment- free follow-up. Ribavirin or placebo treatment assignment was blinded. Sustained virological response was defined as undetectable (less than 50 IU/mL) HCV RNA on or after study week 68. PEGASYS® in combination with ribavirin resulted in a higher SVR compared to PEGASYS® alone or interferon alfa-2b and ribavirin (Table 9). In all treatment arms, patients with viral genotype 1, regardless of viral load, had a lower response rate to PEGASYS® in combination with ribavirin compared to patients with other viral genotypes.
Table 9 Sustained Virologic Response (SVR) to Combination Therapy (Study NV15801)|
Interferon alfa-2b + |
PEGASYS® + |
PEGASYS® + Ribavirin | |
|---|---|---|---|
|
All patients |
197/444 (44%) |
65/224 (29%) |
241/453 (53%) |
|
Genotype 1 |
103/285 (36%) |
29/145 (20%) |
132/298 (44%) |
|
Genotypes 2 to 6 |
94/159 (59%) |
36/79 (46%) |
109/155 (70%) |
Difference in overall treatment response (PEGASYS®/ribavirin – Interferon alfa-2b/ribavirin) was 9% (95% CI 2.3, 15.3).
In Study NV15942, all patients received PEGASYS® 180 mcg subcutaneous once weekly and were randomized to treatment for either 24 or 48 weeks and to a ribavirin dose of either 800 mg or 1000 mg/1200 mg (for body weight less than 75 kg/greater than or equal to 75 kg). Assignment to the four treatment arms was stratified by viral genotype and baseline HCV viral titer. Patients with genotype 1 and high viral titer (defined as greater than 2 x 106 HCV RNA copies/mL serum) were preferentially assigned to treatment for 48 weeks.
** Sustained Virologic Response (SVR) and HCV Genotype**
HCV 1 and 4 - Irrespective of baseline viral titer, treatment for 48 weeks with PEGASYS® and 1000 mg or 1200 mg of ribavirin resulted in higher SVR (defined as undetectable HCV RNA at the end of the 24-week treatment-free follow-up period) compared to shorter treatment (24 weeks) and/or 800 mg ribavirin.
HCV 2 and 3 - Irrespective of baseline viral titer, treatment for 24 weeks with PEGASYS® and 800 mg of ribavirin resulted in a similar SVR compared to longer treatment (48 weeks) and/or 1000 mg or 1200 mg of ribavirin (see Table 10).
The numbers of patients with genotype 5 and 6 were too few to allow for meaningful assessment.
Table 10 Sustained Virologic Response as a Function of Genotype (Study NV15942)|
24 Weeks Treatment |
48 Weeks Treatment | |||
|---|---|---|---|---|
|
PEGASYS® + |
PEGASYS® + |
PEGASYS® + |
PEGASYS® + | |
| ||||
|
Genotype 1 |
29/101 (29%) |
48/118 (41%) |
99/250 (40%) |
138/271 (51%) |
|
Genotypes 2, 3 |
79/96 (82%) |
116/144 (81%) |
75/99 (76%) |
117/153 (76%) |
|
Genotype 4 |
0/5 (0%) |
7/12 (58%) |
5/8 (63%) |
9/11 (82%) |
Pediatric Patients
Previously untreated pediatric subjects 5 through 17 years of age (55% less than 12 years old) with chronic hepatitis C, compensated liver disease and detectable HCV RNA were treated with ribavirin approximately 15 mg/kg/day plus PEGASYS® 180 mcg/1.73 m2 x body surface area once weekly for 48 weeks. All subjects were followed for 24 weeks post-treatment. Sustained virological response (SVR) was defined as undetectable (less than 50 IU/mL) HCV RNA on or after study week 68. A total of 114 subjects were randomized to receive either combination treatment of ribavirin plus PEGASYS® or PEGASYS® monotherapy; subjects failing PEGASYS® monotherapy at 24 weeks or later could receive open- label ribavirin plus PEGASYS®. The initial randomized arms were balanced for demographic factors; 55 subjects received initial combination treatment of ribavirin plus PEGASYS® and 59 received PEGASYS® plus placebo; in the overall intent-to-treat population, 45% were female, 80% were Caucasian, and 81% were infected with HCV genotype 1. The SVR results are summarized inTable 11.
Table 11 Sustained Virologic Response (Study NV17424)|
*Results indicate undetectable HCV RNA defined as HCV RNA less than 50 IU/mL at 24 weeks post-treatment using the AMPLICOR HCV test v2**Scheduled treatment duration was 48 weeks regardless of the genotype | ||
|
PEGASYS**®** |
PEGASYS**®****** | |
|
All HCV genotypes** |
29 (53%) |
12 (20%) |
|
HCV genotype 1 |
21/45 (47%) |
8/47 (17%) |
|
HCV non-genotype 1*** |
8/10 (80%) |
4/12 (33%) |
14.2 Other Treatment Response Predictors
Treatment response rates are lower in patients with poor prognostic factors receiving pegylated interferon alpha therapy. In studies NV15801 and NV15942, treatment response rates were lower in patients older than 40 years (50% vs. 66%), in patients with cirrhosis (47% vs. 59%), in patients weighing over 85 kg (49% vs. 60%), and in patients with genotype 1 with high vs. low viral load (43% vs. 56%). African-American patients had lower response rates compared to Caucasians.
In studies NV15801 and NV15942, lack of early virologic response by 12 weeks (defined as HCV RNA undetectable or greater than 2 log10 lower than baseline) was grounds for discontinuation of treatment. Of patients who lacked an early viral response by 12 weeks and completed a recommended course of therapy despite a protocol-defined option to discontinue therapy, 5/39 (13%) achieved an SVR. Of patients who lacked an early viral response by 24 weeks, 19 completed a full course of therapy and none achieved an SVR.
14.3 Chronic Hepatitis C/HIV Coinfected Patients
In Study NR15961, patients with CHC/HIV were randomized to receive either PEGASYS® 180 mcg subcutaneous once weekly plus an oral placebo, PEGASYS® 180 mcg once weekly plus ribavirin 800 mg by mouth daily or interferon alfa-2a, 3 MIU subcutaneous three times a week plus ribavirin 800 mg by mouth daily. All patients received 48 weeks of therapy and sustained virologic response (SVR) was assessed at 24 weeks of treatment-free follow-up. Ribavirin or placebo treatment assignment was blinded in the PEGASYS® treatment arms. All patients were adults, had compensated liver disease, detectable hepatitis C virus, liver biopsy diagnosis of chronic hepatitis C, and were previously untreated with interferon. Patients also had CD4+ cell count greater than or equal to 200 cells/mm3 or CD4+ cell count greater than or equal to 100 cells/mm3 but less than 200 cells/mm3 and HIV-1 RNA less than 5000 copies/mL, and stable status of HIV. Approximately 15% of patients in the study had cirrhosis. Results are shown inTable 12.
Table 12 Sustained Virologic Response in Patients with Chronic Hepatitis C Coinfected With HIV (Study NR15961)|
Interferon alfa-2a + |
PEGASYS® |
PEGASYS® + | |
|---|---|---|---|
|
All patients |
33 (11%) |
58 (20%) |
116 (40%) |
|
Genotype 1 |
12/171 (7%) |
24/175 (14%) |
51/176 (29%) |
|
Genotypes 2, 3 |
18/89 (20%) |
32/90 (36%) |
59/95 (62%) |
Treatment response rates were lower in CHC/HIV patients with poor prognostic factors (including HCV genotype 1, HCV RNA greater than 800,000 IU/mL, and cirrhosis) receiving pegylated interferon alpha therapy.
Of the patients who did not demonstrate either undetectable HCV RNA or at least a 2 log10 reduction from baseline in HCV RNA titer by 12 weeks of PEGASYS® and ribavirin combination therapy, 2% (2/85) achieved an SVR.
In CHC patients with HIV coinfection who received 48 weeks of PEGASYS® alone or in combination with ribavirin treatment, mean and median HIV RNA titers did not increase above baseline during treatment or 24 weeks post-treatment.
Information For Patients Section
17 PATIENT COUNSELING INFORMATION
- See FDA-approved patient labeling (Medication Guide)
Pregnancy
Patients must be informed that ribavirin may cause birth defects and/or death of the exposed fetus. Ribavirin therapy must not be used by women who are pregnant or by men whose female partners are pregnant. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients taking ribavirin therapy and for 6 months post therapy. Patients should use two reliable methods of birth control while taking ribavirin therapy and for 6 months post therapy. Ribavirin therapy should not be initiated until a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy. Patients must perform a pregnancy test monthly during therapy and for 6 months post therapy.
Female patients of childbearing potential and male patients with female partners of childbearing potential must be advised of the teratogenic/embryocidal risks and must be instructed to practice effective contraception during ribavirin therapy and for 6 months post therapy. Patients should be advised to notify the healthcare provider immediately in the event of a pregnancy [see Contraindications (4) and Warnings and Precautions (5.1)].
** Anemia**
The most common adverse event associated with ribavirin is anemia, which may be severe [see Boxed Warning, Warnings and Precautions (5.2) and Adverse Reactions (6.1)]. Patients should be advised that laboratory evaluations are required prior to starting ribavirin therapy and periodically thereafter [see Warnings and Precautions (5.9)]. It is advised that patients be well hydrated, especially during the initial stages of treatment.
Patients who develop dizziness, confusion, somnolence, and fatigue should be cautioned to avoid driving or operating machinery.
Patients should be advised to take ribavirin with food.
Patients should be questioned about prior history of drug abuse before initiating ribavirin/PEGASYS®, as relapse of drug addiction and drug overdoses have been reported in patients treated with interferons.
Patients should be advised not to drink alcohol, as alcohol may exacerbate chronic hepatitis C infection.
Patients should be informed about what to do in the event they miss a dose of ribavirin. The missed doses should be taken as soon as possible during the same day. Patients should not double the next dose. Patients should be advised to call their healthcare provider if they have questions.
Patients should be informed that the effect of PEGASYS®/ribavirin treatment of hepatitis C infection on transmission is not known, and that appropriate precautions to prevent transmission of hepatitis C virus during treatment or in the event of treatment failure should be taken.
Patients should be informed regarding the potential benefits and risks attendant to the use of ribavirin. Instructions on appropriate use should be given, including review of the contents of the enclosed MEDICATION GUIDE, which is not a disclosure of all or possible adverse effects.
**Dispense with Medication Guide available at: **www.aurobindousa.com/medication-guides
Distributed by:
Aurobindo Pharma USA, Inc.
****279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
****Hyderabad–500 032, India
Revised: 05/2023
**Dispense with Medication Guide available at: **www.aurobindousa.com/medication-guides
