Flucytosine
FLUCYTOSINE Capsules USP Rx only
Approved
Approval ID
ee98e0a9-c125-4b5c-ba4e-ca3c9a1f2b90
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
May 6, 2022
Manufacturers
FDA
Novel Laboratories, Inc.
DUNS: 793518643
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Flucytosine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code40032-771
Application NumberANDA204652
Product Classification
M
Marketing Category
C73584
G
Generic Name
Flucytosine
Product Specifications
Route of AdministrationORAL
Effective DateMay 6, 2022
FDA Product Classification
INGREDIENTS (17)
FLUCYTOSINEActive
Quantity: 250 mg in 1 1
Code: D83282DT06
Classification: ACTIB
LACTOSE MONOHYDRATEInactive
Code: EWQ57Q8I5X
Classification: IACT
SILICON DIOXIDEInactive
Code: ETJ7Z6XBU4
Classification: IACT
MAGNESIUM STEARATEInactive
Code: 70097M6I30
Classification: IACT
FD&C BLUE NO. 1Inactive
Code: H3R47K3TBD
Classification: IACT
TITANIUM DIOXIDEInactive
Code: 15FIX9V2JP
Classification: IACT
TALCInactive
Code: 7SEV7J4R1U
Classification: IACT
GELATINInactive
Code: 2G86QN327L
Classification: IACT
FERROSOFERRIC OXIDEInactive
Code: XM0M87F357
Classification: IACT
SODIUM STARCH GLYCOLATE TYPE A POTATOInactive
Code: 5856J3G2A2
Classification: IACT
WATERInactive
Code: 059QF0KO0R
Classification: IACT
SODIUM LAURYL SULFATEInactive
Code: 368GB5141J
Classification: IACT
SHELLACInactive
Code: 46N107B71O
Classification: IACT
POTASSIUM HYDROXIDEInactive
Code: WZH3C48M4T
Classification: IACT
POVIDONEInactive
Code: FZ989GH94E
Classification: IACT
SODIUM HYDROXIDEInactive
Code: 55X04QC32I
Classification: IACT
FD&C RED NO. 40Inactive
Code: WZB9127XOA
Classification: IACT
Flucytosine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code40032-770
Application NumberANDA204652
Product Classification
M
Marketing Category
C73584
G
Generic Name
Flucytosine
Product Specifications
Route of AdministrationORAL
Effective DateMay 6, 2022
FDA Product Classification
INGREDIENTS (15)
FLUCYTOSINEActive
Quantity: 500 mg in 1 1
Code: D83282DT06
Classification: ACTIB
LACTOSE MONOHYDRATEInactive
Code: EWQ57Q8I5X
Classification: IACT
SILICON DIOXIDEInactive
Code: ETJ7Z6XBU4
Classification: IACT
MAGNESIUM STEARATEInactive
Code: 70097M6I30
Classification: IACT
SODIUM STARCH GLYCOLATE TYPE A POTATOInactive
Code: 5856J3G2A2
Classification: IACT
GELATINInactive
Code: 2G86QN327L
Classification: IACT
TALCInactive
Code: 7SEV7J4R1U
Classification: IACT
TITANIUM DIOXIDEInactive
Code: 15FIX9V2JP
Classification: IACT
FERROSOFERRIC OXIDEInactive
Code: XM0M87F357
Classification: IACT
WATERInactive
Code: 059QF0KO0R
Classification: IACT
SHELLACInactive
Code: 46N107B71O
Classification: IACT
POTASSIUM HYDROXIDEInactive
Code: WZH3C48M4T
Classification: IACT
POVIDONEInactive
Code: FZ989GH94E
Classification: IACT
SODIUM HYDROXIDEInactive
Code: 55X04QC32I
Classification: IACT
FD&C RED NO. 40Inactive
Code: WZB9127XOA
Classification: IACT
Drug Labeling Information
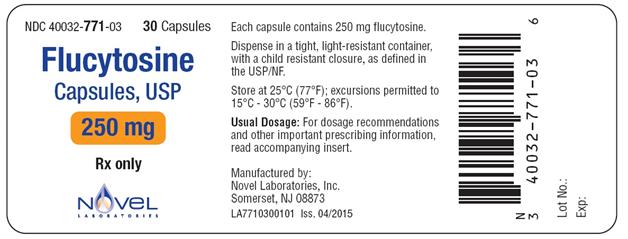
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 4/22/2015
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Flucytosine Capsules USP, 250 mg
30 Capsules
Flucytosine Capsules USP, 500 mg
100 Capsules

