ISENTRESS
These highlights do not include all the information needed to use ISENTRESS safely and effectively. See full prescribing information for ISENTRESS. ISENTRESS (raltegravir) film-coated tablets, for oral useISENTRESS HD (raltegravir) film-coated tablets, for oral useISENTRESS (raltegravir) chewable tablets, for oral useISENTRESS (raltegravir) for oral suspensionInitial U.S. Approval: 2007
89a5ec53-d956-4329-8004-0f40f51c88a3
HUMAN PRESCRIPTION DRUG LABEL
Nov 15, 2023
Merck Sharp & Dohme LLC
DUNS: 118446553
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
RALTEGRAVIR
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
RALTEGRAVIR
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (19)
RALTEGRAVIR
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (21)
RALTEGRAVIR
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (20)
RALTEGRAVIR
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
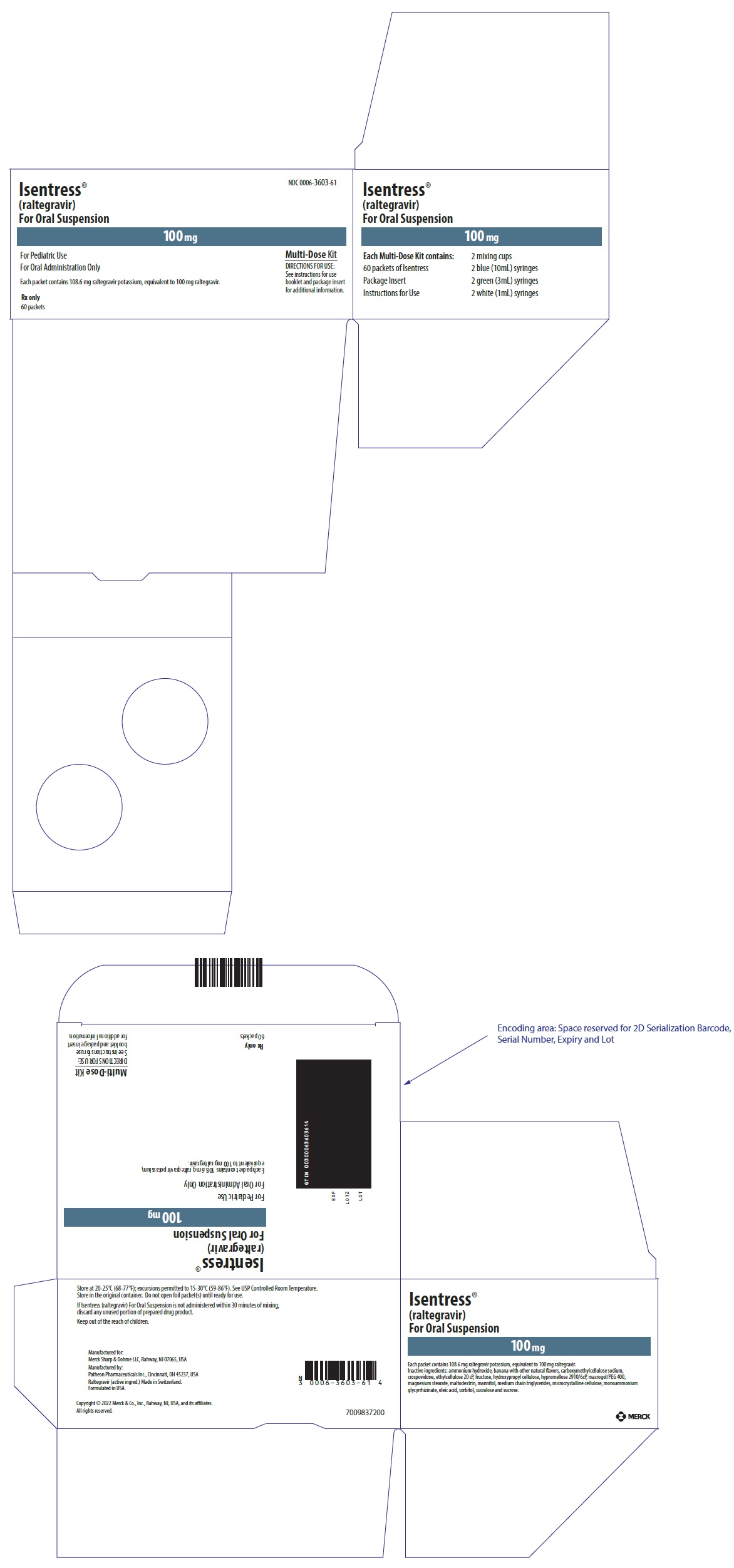
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 mg Packet Carton
NDC 0006-3603-61
Isentress®
(raltegravir)
For Oral Suspension
100 mg
For Pediatric Use
For Oral Administration Only
Each packet contains 108.6 mg raltegravir potassium, equivalent to 100 mg raltegravir.
Rx only
60 packets
Multi-Dose Kit
DIRECTIONS FOR USE:
See instructions for use
booklet and package insert
for additional information.
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience
Treatment-Naïve Adults
The safety of ISENTRESS was evaluated in HIV-infected treatment-naïve subjects in 2 Phase III studies: STARTMRK evaluated ISENTRESS 400 mg twice daily versus efavirenz, both in combination with emtricitabine (+) tenofovir disoproxil fumarate (TDF), and ONCEMRK evaluated ISENTRESS HD 1200 mg (2 × 600 mg) once daily versus ISENTRESS 400 mg twice daily, both in combination with emtricitabine (+) tenofovir disoproxil fumarate. Safety data from these two studies are presented side-by-side in Tables 6 and 7 to simplify presentation; direct comparisons across trials should not be made due to differing duration of follow-up and study design.
STARTMRK (ISENTRESS 400 mg twice daily)
In STARTMRK, subjects received ISENTRESS 400 mg twice daily (N=281) or efavirenz (EFV) 600 mg at bedtime (N=282) both in combination with emtricitabine (+) tenofovir disoproxil fumarate, (N=282). During double-blind treatment, the total follow-up for subjects receiving ISENTRESS 400 mg twice daily + emtricitabine (+) tenofovir disoproxil fumarate was 1104 patient-years and 1036 patient-years for subjects receiving efavirenz 600 mg at bedtime + emtricitabine (+) tenofovir disoproxil fumarate.
In STARTMRK, the rate of discontinuation of therapy due to adverse events through Week 240 was 5% in subjects receiving ISENTRESS + emtricitabine (+) tenofovir disoproxil fumarate and 10% in subjects receiving efavirenz + emtricitabine (+) tenofovir disoproxil fumarate.
ONCEMRK (ISENTRESS HD 1200 mg [2 × 600 mg] once daily)
In ONCEMRK, subjects received ISENTRESS HD 1200 mg once daily (n=531) or ISENTRESS 400 mg twice daily (n=266) both in combination with emtricitabine (+) tenofovir disoproxil fumarate. During double-blind treatment, the total follow-up for subjects with ISENTRESS HD 1200 mg once daily was 913 patient- years and for ISENTRESS 400 mg twice daily was 450 patient-years.
In ONCEMRK, the rate of discontinuation of therapy due to adverse events through Week 96 was 1% in subjects receiving ISENTRESS HD 1200 mg (2 × 600 mg) once daily and 2% in subjects receiving ISENTRESS 400 mg twice daily.
Clinical adverse reactions of moderate to severe intensity occurring in ≥2% of treatment-naïve subjects treated with ISENTRESS 400 mg twice daily or efavirenz in STARTMRK through Week 240 or ISENTRESS HD 1200 mg once daily or ISENTRESS 400 mg twice daily in ONCEMRK through Week 96 are presented in Table 6.
In STARTMRK, clinical adverse reactions of all intensities (mild, moderate and severe) occurring in ≥2% of subjects on ISENTRESS 400 mg twice daily through Week 240 also include diarrhea, flatulence, asthenia, decreased appetite, abnormal dreams, depression and nightmare. In ONCEMRK, clinical adverse reactions of all intensities (mild, moderate and severe) occurring in ≥2% of subjects on ISENTRESS HD or ISENTRESS 400 mg twice daily through Week 96 also include abdominal pain, diarrhea, vomiting, and decreased appetite.
Table 6: Adverse Reactions* of Moderate to Severe Intensity† Occurring in ≥2% of Treatment-Naïve Adult Subjects Receiving ISENTRESS and ISENTRESS HD|
System Organ Class, Preferred Term |
STARTMRK |
ONCEMRK | ||
|---|---|---|---|---|
|
ISENTRESS 400 mg Twice Daily |
Efavirenz 600 mg At Bedtime |
ISENTRESS HD 1200 mg Once Daily |
ISENTRESS 400 mg Twice Daily | |
|
Note: ISENTRESS BID, ISENTRESS HD and efavirenz were administered with emtricitabine (+) tenofovir disoproxil fumarate | ||||
|
N= total number of subjects per treatment group | ||||
| ||||
|
Headache |
4% |
5% |
1% |
<1% |
|
Insomnia |
4% |
4% |
<1% |
<1% |
|
Nausea |
3% |
4% |
1% |
0% |
|
Dizziness |
2% |
6% |
<1% |
0% |
|
Fatigue |
2% |
3% |
0% |
0% |
Laboratory Abnormalities
The percentages of adult subjects with selected Grade 2 to 4 laboratory abnormalities (that represent a worsening Grade from baseline) who were treated with ISENTRESS 400 mg twice daily or efavirenz in STARTMRK or ISENTRESS HD 1200 mg once daily or ISENTRESS 400 mg twice daily in ONCEMRK are presented in Table 7.
Table 7: Selected Grade 2 to 4 Laboratory Abnormalities Reported in Treatment-Naïve Subjects|
STARTMRK |
ONCEMRK | ||||
|---|---|---|---|---|---|
|
Laboratory Parameter Preferred Term (Unit) |
Limit |
ISENTRESS |
Efavirenz |
ISENTRESS HD |
ISENTRESS |
|
ULN = Upper limit of normal range | |||||
|
Notes: ISENTRESS BID, ISENTRESS HD and Efavirenz were administered with emtricitabine (+) tenofovir disoproxil fumarate | |||||
| |||||
|
Hematology | |||||
|
Absolute neutrophil count (103/µL) | |||||
|
Grade 2 |
0.75 - 0.999 |
3% |
5% |
2% |
1% |
|
Grade 3 |
0.50 - 0.749 |
3% |
1% |
1% |
1% |
|
Grade 4 |
<0.50 |
1% |
1% |
<1% |
0% |
|
Hemoglobin (gm/dL) | |||||
|
Grade 2 |
7.5 - 8.4 |
1% |
1% |
0% |
0% |
|
Grade 3 |
6.5 - 7.4 |
1% |
1% |
0% |
0% |
|
Grade 4 |
<6.5 |
<1% |
0% |
0% |
0% |
|
Platelet count (103/µL) | |||||
|
Grade 2 |
50 - 99.999 |
1% |
0% |
1% |
<1% |
|
Grade 3 |
25 - 49.999 |
<1% |
<1% |
0% |
0% |
|
Grade 4 |
<25 |
0% |
0% |
0% |
<1% |
|
Blood chemistry | |||||
|
Fasting (non-random) serum glucose test (mg/dL)* | |||||
|
Grade 2 |
126 - 250 |
7% |
6% |
|
|
|
Grade 3 |
251 - 500 |
2% |
1% |
|
|
|
Grade 4 |
|
0% |
0% |
|
|
|
Total serum bilirubin | |||||
|
Grade 2 |
1.6 - 2.5 × ULN |
5% |
<1% |
3% |
2% |
|
Grade 3 |
2.6 - 5.0 × ULN |
1% |
0% |
1% |
<1% |
|
Grade 4 |
|
<1% |
0% |
<1% |
0% |
|
Creatinine | |||||
|
Grade 2 |
1.4-1.8 × ULN |
1% |
1% |
0% |
<1% |
|
Grade 3 |
1.9-3.4 × ULN |
0% |
<1% |
0% |
0% |
|
Grade 4 |
≥3.5 × ULN |
0% |
0% |
0% |
0% |
|
Serum aspartate aminotransferase | |||||
|
Grade 2 |
2.6 - 5.0 × ULN |
8% |
10% |
5% |
3% |
|
Grade 3 |
5.1 - 10.0 × ULN |
5% |
3% |
2% |
<1% |
|
Grade 4 |
|
1% |
<1% |
1% |
<1% |
|
Serum alanine aminotransferase | |||||
|
Grade 2 |
2.6 - 5.0 × ULN |
11% |
12% |
4% |
2% |
|
Grade 3 |
5.1 - 10.0 × ULN |
2% |
2% |
1% |
<1% |
|
Grade 4 |
|
2% |
1% |
1% |
<1% |
|
Serum alkaline phosphatase | |||||
|
Grade 2 |
2.6 - 5.0 × ULN |
1% |
3% |
1% |
0% |
|
Grade 3 |
5.1 - 10.0 × ULN |
0% |
1% |
<1% |
0% |
|
Grade 4 |
|
<1% |
<1% |
0% |
0% |
|
Lipase† | |||||
|
Grade 2 |
1.6-3.0 x ULN |
|
|
7% |
5% |
|
Grade 3 |
3.1-5.0 × ULN |
|
|
2% |
1% |
|
Grade 4 |
|
|
|
2% |
1% |
|
Creatine kinase† | |||||
|
Grade 2 |
6.0-9.9 × ULN |
|
|
4% |
5% |
|
Grade 3 |
10.0-19.9 × ULN |
|
|
3% |
3% |
|
Grade 4 |
≥20.0 × ULN |
|
|
3% |
2% |
Lipids, Change from Baseline
Changes from baseline in fasting lipids are shown in Table 8.
Table 8: Lipid Values, Mean Change from Baseline, STARTMRK Study|
Laboratory Parameter Preferred Term |
ISENTRESS 400 mg |
Efavirenz 600 mg | ||||
|---|---|---|---|---|---|---|
|
Change from Baseline at |
Change from Baseline at | |||||
|
Baseline |
Week 240 |
Mean Change |
Baseline |
Week 240 |
Mean Change | |
|
(mg/dL) |
(mg/dL) |
(mg/dL) |
(mg/dL) |
(mg/dL) |
(mg/dL) | |
|
Notes: | ||||||
| ||||||
|
LDL-Cholesterol* |
96 |
106 |
10 |
93 |
118 |
25 |
|
HDL-Cholesterol* |
38 |
44 |
6 |
38 |
51 |
13 |
|
Total Cholesterol* |
159 |
175 |
16 |
157 |
201 |
44 |
|
Triglyceride* |
128 |
130 |
2 |
141 |
178 |
37 |
Treatment-Experienced Adults
The safety assessment of ISENTRESS in treatment-experienced subjects is based on the pooled safety data from the randomized, double-blind, placebo- controlled trials, BENCHMRK 1 and BENCHMRK 2 in antiretroviral treatment- experienced HIV-1 infected adult subjects. A total of 462 subjects received the recommended dose of ISENTRESS 400 mg twice daily in combination with optimized background therapy (OBT) compared to 237 subjects taking placebo in combination with OBT. The median duration of therapy in these trials was 96 weeks for subjects receiving ISENTRESS and 38 weeks for subjects receiving placebo. The total exposure to ISENTRESS was 708 patient-years versus 244 patient-years on placebo. The rates of discontinuation due to adverse events were 4% in subjects receiving ISENTRESS and 5% in subjects receiving placebo.
Clinical ADRs were considered by investigators to be causally related to ISENTRESS + OBT or placebo + OBT. Clinical ADRs of moderate to severe intensity occurring in ≥2% of subjects treated with ISENTRESS and occurring at a higher rate compared to placebo are presented in Table 9.
Table 9: Adverse Drug Reactions* of Moderate to Severe Intensity† Occurring in ≥2% of Treatment-Experienced Adult Subjects Receiving ISENTRESS and at a Higher Rate Compared to Placebo (96 Week Analysis)|
System Organ Class, Adverse Reactions |
Randomized Studies BENCHMRK 1 and BENCHMRK 2 | |
|---|---|---|
|
ISENTRESS 400 mg Twice Daily + OBT |
Placebo + OBT | |
|
Nervous System Disorders | ||
|
n=total number of subjects per treatment group. | ||
| ||
|
Headache |
2% |
<1% |
Laboratory Abnormalities
The percentages of adult subjects treated with ISENTRESS 400 mg twice daily or placebo in Studies BENCHMRK 1 and BENCHMRK 2 with selected Grade 2 to 4 laboratory abnormalities representing a worsening Grade from baseline are presented in Table 10.
Table 10: Selected Grade 2 to 4 Laboratory Abnormalities Reported in Treatment-Experienced Subjects (96 Week Analysis)|
Randomized Studies BENCHMRK 1 and BENCHMRK 2 | |||
|---|---|---|---|
|
Laboratory Parameter Preferred Term |
Limit |
ISENTRESS 400 mg Twice Daily + OBT |
Placebo + OBT |
|
ULN = Upper limit of normal range | |||
|
Hematology | |||
|
Absolute neutrophil count (103/µL) | |||
|
Grade 2 |
0.75 - 0.999 |
4% |
5% |
|
Grade 3 |
0.50 - 0.749 |
3% |
3% |
|
Grade 4 |
<0.50 |
1% |
<1% |
|
Hemoglobin (gm/dL) | |||
|
Grade 2 |
7.5 - 8.4 |
1% |
3% |
|
Grade 3 |
6.5 - 7.4 |
1% |
1% |
|
Grade 4 |
<6.5 |
<1% |
0% |
|
Platelet count (103/µL) | |||
|
Grade 2 |
50 - 99.999 |
3% |
5% |
|
Grade 3 |
25 - 49.999 |
1% |
<1% |
|
Grade 4 |
<25 |
1% |
<1% |
|
Blood chemistry | |||
|
Fasting (non-random) serum glucose test (mg/dL) | |||
|
Grade 2 |
126 - 250 |
10% |
7% |
|
Grade 3 |
251 - 500 |
3% |
1% |
|
Grade 4 |
|
0% |
0% |
|
Total serum bilirubin | |||
|
Grade 2 |
1.6 - 2.5 × ULN |
6% |
3% |
|
Grade 3 |
2.6 - 5.0 × ULN |
3% |
3% |
|
Grade 4 |
|
1% |
0% |
|
Serum aspartate aminotransferase | |||
|
Grade 2 |
2.6 - 5.0 × ULN |
9% |
7% |
|
Grade 3 |
5.1 - 10.0 × ULN |
4% |
3% |
|
Grade 4 |
|
1% |
1% |
|
Serum alanine aminotransferase | |||
|
Grade 2 |
2.6 - 5.0 × ULN |
9% |
9% |
|
Grade 3 |
5.1 - 10.0 × ULN |
4% |
2% |
|
Grade 4 |
|
1% |
2% |
|
Serum alkaline phosphatase | |||
|
Grade 2 |
2.6 - 5.0 × ULN |
2% |
<1% |
|
Grade 3 |
5.1 - 10.0 × ULN |
<1% |
1% |
|
Grade 4 |
|
1% |
<1% |
|
Serum pancreatic amylase test | |||
|
Grade 2 |
1.6 - 2.0 × ULN |
2% |
1% |
|
Grade 3 |
2.1 - 5.0 × ULN |
4% |
3% |
|
Grade 4 |
|
<1% |
<1% |
|
Serum lipase test | |||
|
Grade 2 |
1.6 - 3.0 × ULN |
5% |
4% |
|
Grade 3 |
3.1 - 5.0 × ULN |
2% |
1% |
|
Grade 4 |
|
0% |
0% |
|
Serum creatine kinase | |||
|
Grade 2 |
6.0 - 9.9 × ULN |
2% |
2% |
|
Grade 3 |
10.0 - 19.9 × ULN |
4% |
3% |
|
Grade 4 |
≥20.0 × ULN |
3% |
1% |
Less Common Adverse Reactions Observed in Treatment-Naïve and Treatment- Experienced Studies
The following ADRs occurred in <2% of treatment-naïve or treatment-experienced subjects receiving ISENTRESS or ISENTRESS HD in a combination regimen. These events have been included because of their seriousness, increased frequency compared with efavirenz or placebo, or investigator's assessment of potential causal relationship.
Gastrointestinal Disorders: abdominal pain, gastritis, dyspepsia, vomiting
General Disorders and Administration Site Conditions: asthenia
Hepatobiliary Disorders: hepatitis
Immune System Disorders: hypersensitivity
Infections and Infestations: genital herpes, herpes zoster
Psychiatric Disorders: depression (particularly in subjects with a pre- existing history of psychiatric illness), including suicidal ideation and behaviors
Renal and Urinary Disorders: nephrolithiasis, renal failure
Selected Adverse Events - Adults
In studies of ISENTRESS 400 mg twice daily, cancers were reported in treatment-experienced subjects who initiated ISENTRESS or placebo, both with OBT, and in treatment-naïve subjects who initiated ISENTRESS or efavirenz, both with emtricitabine (+) tenofovir disoproxil fumarate; several were recurrent. The types and rates of specific cancers were those expected in a highly immunodeficient population (many had CD4+ counts below 50 cells/mm3 and most had prior AIDS diagnoses). The risk of developing cancer in these studies was similar in the group receiving ISENTRESS and the group receiving the comparator.
Grade 2-4 creatine kinase laboratory abnormalities were observed in subjects treated with ISENTRESS and ISENTRESS HD (see Tables 6 and 8). Myopathy and rhabdomyolysis have been reported with ISENTRESS. Use with caution in patients at increased risk of myopathy or rhabdomyolysis, such as patients receiving concomitant medications known to cause these conditions and patients with a history of rhabdomyolysis, myopathy or increased serum creatine kinase.
Rash occurred more commonly in treatment-experienced subjects receiving regimens containing ISENTRESS + darunavir/ritonavir compared to subjects receiving ISENTRESS without darunavir/ritonavir or darunavir/ritonavir without ISENTRESS. However, rash that was considered drug related occurred at similar rates for all three groups. These rashes were mild to moderate in severity and did not limit therapy; there were no discontinuations due to rash.
Patients with Co-existing Conditions - Adults
Patients Co-infected with Hepatitis B and/or Hepatitis C Virus
In Phase III studies of ISENTRESS, patients with chronic (but not acute) active hepatitis B and/or hepatitis C virus co-infection were permitted to enroll provided that baseline liver function tests did not exceed 5 times the upper limit of normal (ULN). In the treatment-experienced studies, BENCHMRK 1 and BENCHMRK 2, 16% of all patients (114/699) were co-infected; in the treatment-naïve studies, STARTMRK and ONCEMRK, 6% (34/563) and 3% (23/797), respectively, were co-infected. In general the safety profile of ISENTRESS in subjects with hepatitis B and/or hepatitis C virus co-infection was similar to that in subjects without hepatitis B and/or hepatitis C virus co-infection, although the rates of AST and ALT abnormalities were higher in the subgroup with hepatitis B and/or hepatitis C virus co-infection for all treatment groups.
At 96 weeks, in treatment-experienced subjects receiving ISENTRESS 400 mg twice daily, Grade 2 or higher laboratory abnormalities that represent a worsening Grade from baseline of AST, ALT or total bilirubin occurred in 29%, 34% and 13%, respectively, of co-infected subjects treated with ISENTRESS as compared to 11%, 10% and 9% of all other subjects treated with ISENTRESS. At 240 weeks, in treatment-naïve subjects receiving ISENTRESS 400 mg twice daily, Grade 2 or higher laboratory abnormalities that represent a worsening Grade from baseline of AST, ALT or total bilirubin occurred in 22%, 44% and 17%, respectively, of co-infected subjects treated with ISENTRESS as compared to 13%, 13% and 5% of all other subjects treated with ISENTRESS.
At 96 weeks, in treatment-naïve subjects receiving ISENTRESS HD 1200 mg (2 × 600 mg) once daily, Grade 2 or higher laboratory abnormalities that represent a worsening Grade from baseline of AST, ALT or total bilirubin occurred in 27%, 40% and 13%, respectively, of co-infected subjects treated with ISENTRESS HD 1200 mg once daily as compared to 7%, 5% and 3% of all other subjects treated with ISENTRESS HD 1200 mg once daily.
Pediatrics
2 to 18 Years of Age
ISENTRESS has been studied in 126 antiretroviral treatment-experienced HIV-1 infected children and adolescents 2 to 18 years of age, in combination with other antiretroviral agents in IMPAACT P1066 [see Use in Specific Populations (8.4) and Clinical Studies (14.4)]. Of the 126 patients, 96 received the recommended dose of ISENTRESS.
In these 96 children and adolescents, frequency, type and severity of drug related adverse reactions through Week 24 were comparable to those observed in adults.
One patient experienced drug related clinical adverse reactions of Grade 3 psychomotor hyperactivity, abnormal behavior and insomnia; one patient experienced a Grade 2 serious drug related allergic rash.
One patient experienced drug related laboratory abnormalities, Grade 4 AST and Grade 3 ALT, which were considered serious.
4 Weeks to Less than 2 Years of Age
ISENTRESS has also been studied in 26 HIV-1 infected infants and toddlers 4 weeks to less than 2 years of age, in combination with other antiretroviral agents in IMPAACT P1066 [see Use in Specific Populations (8.4) and Clinical Studies (14.4)].
In these 26 infants and toddlers, the frequency, type and severity of drug related adverse reactions through Week 48 were comparable to those observed in adults.
One patient experienced a Grade 3 serious drug related allergic rash that resulted in treatment discontinuation.
HIV-1 Exposed Neonates
Forty-two neonates were treated with ISENTRESS for up to 6 weeks from birth, and followed for a total of 24 weeks in IMPAACT P1110 [see Use in Specific Populations (8.4)]. There were no drug related clinical adverse reactions and three drug related laboratory adverse reactions (one case of transient Grade 4 neutropenia in a subject receiving zidovudine-containing regimen for prevention of mother to child transmission (PMTCT), and two bilirubin elevations (one each, Grade 1 and Grade 2) considered non-serious and not requiring specific therapy). The safety profile in neonates was generally similar to that observed in older patients treated with ISENTRESS. No clinically meaningful differences in the adverse event profile of neonates were observed when compared to adults.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ISENTRESS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: thrombocytopenia
Gastrointestinal Disorders: diarrhea
Hepatobiliary Disorders: hepatic failure (with and without associated hypersensitivity) in patients with underlying liver disease and/or concomitant medications
Musculoskeletal and Connective Tissue Disorders: rhabdomyolysis
Nervous System Disorders: cerebellar ataxia
Psychiatric Disorders: anxiety, paranoia
- The most common adverse reactions of moderate to severe intensity (≥2%) are insomnia, headache, dizziness, nausea and fatigue (6.1).
- Creatine kinase elevations were observed in subjects who received ISENTRESS or ISENTRESS HD. Myopathy and rhabdomyolysis have been reported. Use with caution in patients at increased risk of myopathy or rhabdomyolysis, such as patients receiving concomitant medications known to cause these conditions and patients with a history of rhabdomyolysis, myopathy or increased serum creatine kinase (6.2).
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
11 DESCRIPTION
ISENTRESS contains raltegravir potassium, a human immunodeficiency virus integrase strand transfer inhibitor. The chemical name for raltegravir potassium is N-[(4-Fluorophenyl) methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-4-pyrimidinecarboxamide monopotassium salt.
The empirical formula is C20H20FKN6O5 and the molecular weight is 482.51. The structural formula is:
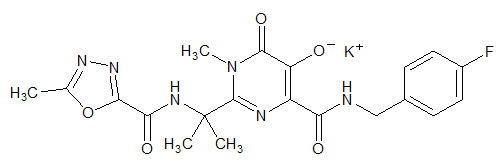
Raltegravir potassium is a white to off-white powder. It is soluble in water, slightly soluble in methanol, very slightly soluble in ethanol and acetonitrile and insoluble in isopropanol.
Each 400 mg film-coated tablet of ISENTRESS for oral administration contains 434.4 mg of raltegravir (as potassium salt), equivalent to 400 mg of raltegravir free phenol and the following inactive ingredients: calcium phosphate dibasic anhydrous, hypromellose 2208, lactose monohydrate, magnesium stearate, microcrystalline cellulose, poloxamer 407 (contains 0.01% butylated hydroxytoluene as antioxidant), sodium stearyl fumarate. In addition, the film coating contains the following inactive ingredients: black iron oxide, polyethylene glycol 3350, polyvinyl alcohol, red iron oxide, talc and titanium dioxide.
Each 600 mg film-coated tablet of ISENTRESS HD for oral administration contains 651.6 mg of raltegravir (as potassium salt), equivalent to 600 mg of raltegravir free phenol and the following inactive ingredients: croscarmellose sodium, hypromellose 2910, magnesium stearate, microcrystalline cellulose. The film coating contains the following inactive ingredients: ferrosoferric oxide, hypromellose 2910, iron oxide yellow, lactose monohydrate, triacetin and titanium dioxide. The tablet may also contain trace amount of carnauba wax.
Each 100 mg chewable tablet of ISENTRESS for oral administration contains 108.6 mg of raltegravir (as potassium salt), equivalent to 100 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, magnesium stearate, mannitol, medium chain triglycerides, monoammonium glycyrrhizinate, natural and artificial flavors (orange, banana, and masking that contains aspartame), oleic acid, PEG 400, red iron oxide, saccharin sodium, sodium citrate dihydrate, sodium stearyl fumarate, sorbitol, sucralose and yellow iron oxide.
Each 25 mg chewable tablet of ISENTRESS for oral administration contains 27.16 mg of raltegravir (as potassium salt), equivalent to 25 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, magnesium stearate, mannitol, medium chain triglycerides, monoammonium glycyrrhizinate, natural and artificial flavors (orange, banana, and masking that contains aspartame), oleic acid, PEG 400, saccharin sodium, sodium citrate dihydrate, sodium stearyl fumarate, sorbitol, sucralose and yellow iron oxide.
Each packet of ISENTRESS for oral suspension 100 mg, contains 108.6 mg of raltegravir (as potassium salt), equivalent to 100 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, banana with other natural flavors, carboxymethylcellulose sodium, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, macrogol/PEG 400, magnesium stearate, maltodextrin, mannitol, medium chain triglycerides, microcrystalline cellulose, monoammonium glycyrrhizinate, oleic acid, sorbitol, sucralose and sucrose.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies of raltegravir in mice did not show any carcinogenic potential. At the highest dose levels, 400 mg/kg/day in females and 250 mg/kg/day in males, systemic exposure was 1.8-fold (females) or 1.2-fold (males) greater than the AUC (54 µM∙hr) at the 400-mg twice daily human dose. Treatment-related squamous cell carcinoma of nose/nasopharynx was observed in female rats dosed with 600 mg/kg/day raltegravir for 104 weeks. These tumors were possibly the result of local irritation and inflammation due to local deposition and/or aspiration of drug in the mucosa of the nose/nasopharynx during dosing. No tumors of the nose/nasopharynx were observed in rats dosed with 150 mg/kg/day (males) and 50 mg/kg/day (females) and the systemic exposure in rats was 1.7-fold (males) to 1.4-fold (females) greater than the AUC (54 µM∙hr) at the 400-mg twice daily human dose.
No evidence of mutagenicity or genotoxicity was observed in in vitro microbial mutagenesis (Ames) tests, in vitro alkaline elution assays for DNA breakage, and in vitro and in vivo chromosomal aberration studies.
No effect on fertility was seen in male and female rats at doses up to 600 mg/kg/day which resulted in a 3-fold exposure above the exposure at the recommended human dose.
INSTRUCTIONS FOR USE SECTION
Bring this booklet to your child's appointments.
ISENTRESS (raltegravir)
for oral suspension
Instructions for Use
for babies and toddlers
Before You Start
|
Note: Make sure your doctor shows you how to prepare and give ISENTRESS for oral suspension. |
-
Be sure you understand these instructions before you start. Call your doctor if you have any questions.
-
It is very important that you measure the water and ISENTRESS carefully using the correct syringe.
-
Before you give ISENTRESS to your child, check the expiration date. The expiration date is printed on the box and the ISENTRESS packets.
-
Do not open the ISENTRESS packets until you are ready to mix a dose.
-
The amount of ISENTRESS depends on your child's age and weight, so it will change over time.
Your doctor will tell you the right dose at each check-up after weighing your child.
Be sure to keep your doctor's appointments so you get new dosing information as your child grows.
During your child's first week of life, you will give ISENTRESS 1 time a day. After the first week of life, you will give it 2 times a day. -
This booklet tells you how to:
- Mix ISENTRESS into a liquid form
- Measure the right dose using a syringe
- Give mixed ISENTRESS to your child
- Clean up
Kit Contents
|
|
|
|
|
|
|
|
|
|
|
|
|
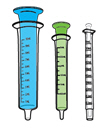
2blue (10mL) syringes |
2green (3mL) syringes |
2white (1mL) syringes |
The kit has an extra cup and set of syringes in case one is lost or damaged.
Do not use any damaged cups or syringes.
Step 1. Get ready
- Put your child in a safe place. You will need both hands to prepare ISENTRESS.
- Wash your hands with soap and water.
- Take out what you need from the kit as shown below to make 1 dose and place on a clean surface:
|
|
|
|
|
|
1 mixing cup |
1 packet of ISENTRESS |
a clean glass |
3 syringes |
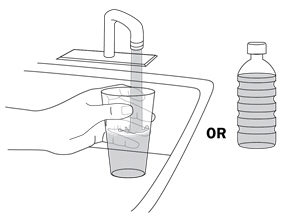
Step 2. Fill a clean glass with water
|
Fill a clean glass with room-temperature drinking water from your sink or use bottled water. |
|
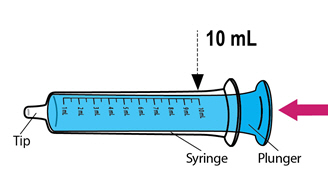
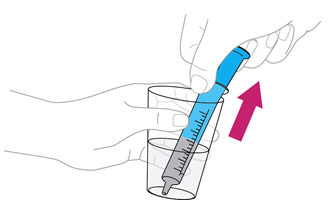
Step 3. Fill theblue**** syringe with water
|
Push the plunger of theblue syringe into the syringe as far as it goes. |
|
|
Put the tip of the syringe into the glass of water. Pull back the plunger to draw up water into the syringe. Stop when you get to the 10mL mark. |
|
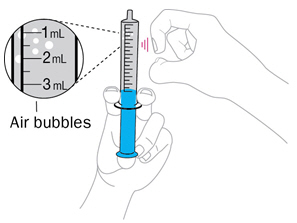
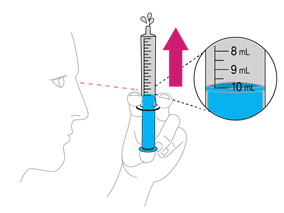
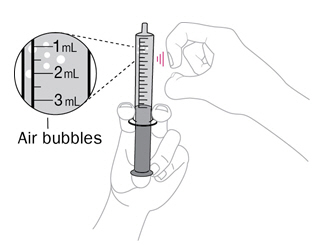
Step 4. Check for air bubbles
|
Hold the syringe with tip up. Tap it with your finger to move any air bubbles up towards the tip. Slowly push up on the plunger to make the air come out. You may see some drops of water come out. |
|
|
|
Re-check the amount of water in the syringe. If it is less than 10mL, put the tip back into the water and pull back on the plunger until you get to the 10mL mark. |
Step 5. Add the 10mL of water to the mixing cup
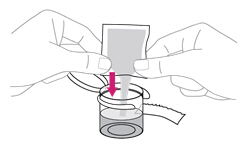
Add the 10 mL of water from the syringe to the mixing cup by pushing all the way down on the plunger.
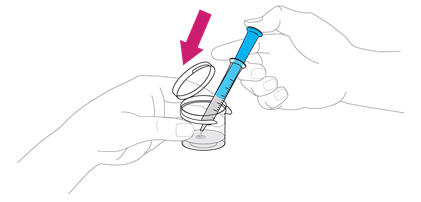
Step 6. Add ISENTRESS to the mixing cup
|
Note before adding ISENTRESS: |
|
|
Take 1 packet of ISENTRESS and shake the powder to the bottom of the packet. |
|
Tear or cut open the packet at the dotted line and add all of the powder to the water in the mixing cup. Make sure the packet is completely empty. |
|
Step 7. Mix ISENTRESS and water
|
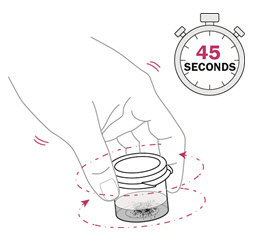
Snap the lid of the mixing cup shut. Gently swirl the mixing cup for 45 seconds in a circular motion to mix the powder and water. Use a clock or timer to time for 45 seconds.Do not shake the mixture. |
|
|
|
Check to make sure the powder is mixed. If it is not mixed, swirl it some more. The mixture should look cloudy. |
Step 8. Check your prescription
Find the dose amount in 'mL' prescribed by your doctor. This is written on the prescription label on the box from your pharmacy.
Remember that the dose may change each time you go to the doctor, so make sure you check the prescribed dose each time. Be sure to go to all of your doctor's appointments so your child gets the right dose!
Step 9. Choose the syringe you need
Your doctor will prescribe the dose in milliliters (mL). Choose the right syringe for your child's dose:
|
WHITE |
GREEN |
|
BLUE |
|
Then find the mL mark on the syringe that matches your child's dose.
Step 10. Measure ISENTRESS
|
Push the plunger into the barrel of the syringe as far as it goes. |
|
|
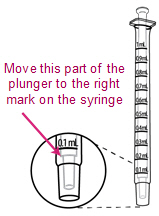
Put the tip of the syringe into the cup of the mixed ISENTRESS and pull back on the plunger. Stop when you get to the line that matches your child's prescribed dose. |
|
|
IMPORTANT:
|
Step 11. Check for air bubbles
|
Hold the syringe with tip up. Tap it with your finger to move any air bubbles up towards the tip. Slowly push the plunger to make the air come out. You may see some drops of medicine come out. |
|
|
|
Re-check the amount of ISENTRESS in the syringe. If it is less than the prescribed dose, put the tip back into the cup of mixed ISENTRESS and pull back on the plunger until you get to the right dose mark. |
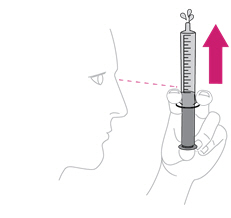
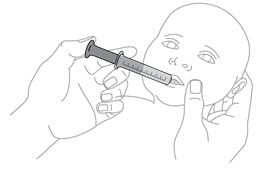
Step 12. Give ISENTRESS to your child
|
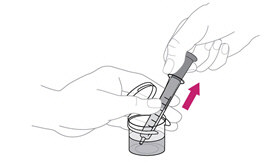
Place the tip of the syringe inside your child's mouth so that it touches either the right or left cheek. |
|
Slowly push in the plunger to give the dose of mixed ISENTRESS to your child. If your child fusses, take the tip of the syringe out of the mouth and try again. It is important that your child takes all of the prescribed dose (a little left in the syringe tip is OK).
|
IMPORTANT: |
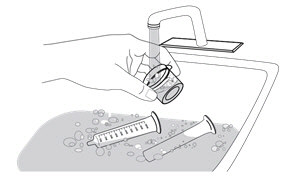
Step 13. Clean up
Pour the leftover mixed ISENTRESS into the trash.
Do not pour it into the sink.
Pull the plungers out of any syringes you used.
Hand wash the syringes, plungers, and mixing cup with warm water and dish soap. Do not wash in the dishwasher.
|
Rinse with water and let air dry. Put everything in a clean, dry place. |
|
How should I store ISENTRESS?
Store theISENTRESS for oral suspension kit at room temperature between 68°F to 77°F (20°C to 25°C).
Store in the original container.
Keep ISENTRESS and all medicines out of the reach of children.
|
Be sure to keep your doctor's appointments so you always know how much ISENTRESS to give to your child. |
For more information, go to www.ISENTRESS.com or call 1-800-622-4477.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Recommendations
- Because the formulations have different pharmacokinetic profiles, do not substitute ISENTRESS chewable tablets or ISENTRESS for oral suspension for the ISENTRESS 400 mg film-coated tablet or the ISENTRESS HD 600 mg film-coated tablet. See specific dosing guidance for chewable tablets and the formulation for oral suspension.
- Because the extent to which ISENTRESS may be dialyzable is unknown, dosing before a dialysis session should be avoided [see Clinical Pharmacology (12.3)].
- ISENTRESS film-coated tablets must be swallowed whole.
- ISENTRESS chewable tablets may be chewed or swallowed whole. Maximum daily dose is 300 mg taken by mouth twice daily.
- For children who have difficulty chewing the 25 mg chewable tablet, the tablet may be crushed.
- Preparation of the crushed 25 mg chewable tablet:
- Place the tablet(s) in a small, clean cup. For each tablet, add a teaspoonful (~5 mL) of liquid (for example, water, juice, or breast milk).
- Within 2 minutes, the tablet(s) will absorb the liquid and fall apart.
- Using a spoon, crush any remaining pieces of the tablet(s). Immediately administer the entire dose orally.
- If any portion of the dose is left in the cup, add another teaspoonful (~5 mL) of liquid, swirl and administer immediately.
- Preparation of the crushed 25 mg chewable tablet:
- ISENTRESS for oral suspension:
- SeeInstructions for Use for details on preparation and administration of ISENTRESS for oral suspension.
- Using the provided mixing cup, combine 10 mL of water and the entire contents of one packet of ISENTRESS for oral suspension and mix. Each single-use packet for oral suspension contains 100 mg of raltegravir which is suspended in 10 mL of water giving a final concentration of 10 mg per mL. Maximum daily dose is 100 mg taken by mouth twice daily.
- Gently swirl the mixing cup for 45 seconds in a circular motion to mix the powder into a uniform suspension.Do not shake.
- Once mixed, measure the prescribed dose volume of suspension with a syringe and administer the dose orally. The dose should be administered orally within 30 minutes of mixing.
- Discard any remaining suspension into the trash.
2.2 Adults
The recommended adult dosage of ISENTRESS film-coated tablets is displayed in Table 1. ISENTRESS and ISENTRESS HD should be taken by mouth and may be taken with or without food [see Clinical Pharmacology (12.3)].
Table 1: Dosing Recommendations for ISENTRESS and ISENTRESS HD in Adult Patients|
Population |
Recommended Dose |
|---|---|
|
Treatment-naïve patients or patients who are virologically suppressed on an initial regimen of ISENTRESS 400 mg twice daily |
1200 mg (2 × 600 mg) once daily |
|
Treatment-experienced |
400 mg twice daily |
|
Treatment-naïve or treatment-experienced when coadministered with rifampin [see Drug Interactions (7.1)] |
800 mg (2 × 400 mg) twice daily |
2.3 Pediatrics
The recommended pediatric dosage of ISENTRESS is displayed in Table 2. ISENTRESS film-coated tablets, chewable tablets and for oral suspension should be taken by mouth and may be taken with or without food [see Clinical Pharmacology (12.3)].
Table 2: Dosing Recommendations for ISENTRESS and ISENTRESS HD in Pediatric Patients|
Recommended Pediatric Dosage and Formulation | ||||
|---|---|---|---|---|
|
Population/Weight |
Film-Coated Tablets 400 mg |
Film-Coated Tablets 600 mg |
Chewable Tablets 100 mg and 25 mg |
For Oral Suspension 100 mg |
| ||||
|
If at least 40 kg and either:
|
400 mg twice daily |
1200 mg (2 × 600 mg) once daily |
300 mg twice daily (see Table 3) |
NA |
|
If at least 25 kg |
400 mg twice daily* |
NA |
Weight-based dosing twice daily (see Table 3) |
NA |
|
If at least 4 weeks of age and weighing 3 kg to less than 25 kg |
NA |
NA |
Weight-based dosing twice daily (see Table 4) |
Weight-based dosing twice daily up to 20 kg (see Table 4) |
|
From birth to 4 weeks (28 days) weighing at least 2 kg |
NA |
NA |
NA |
Weight-based dosing once daily or twice daily (see Table 5) |
|
Body Weight |
Dose |
Number of 100 mg Chewable Tablets |
|---|---|---|
| ||
|
25 to less than 28 |
150 mg twice daily |
1.5 × 100 mg† twice daily |
|
28 to less than 40 |
200 mg twice daily |
2 × 100 mg twice daily |
|
At least 40 |
300 mg twice daily |
3 × 100 mg twice daily |
|
Body Weight |
Volume (Dose) of Suspension to be Administered |
Number of Chewable Tablets† |
|---|---|---|
| ||
|
3 to less than 4 |
2.5 mL (25 mg) twice daily |
1 × 25 mg twice daily‡ |
|
4 to less than 6 |
3 mL (30 mg) twice daily | |
|
6 to less than 8 |
4 mL (40 mg) twice daily |
2 × 25 mg twice daily‡ |
|
8 to less than 10 |
6 mL (60 mg) twice daily | |
|
10 to less than 14 |
8 mL (80 mg) twice daily |
3 × 25 mg twice daily‡ |
|
14 to less than 20 |
10 mL (100 mg) twice daily |
1 × 100 mg twice daily |
|
20 to less than 25 |
Not applicable |
1.5 × 100 mg§ twice daily |
- For full-term neonates (birth to 4 weeks [28 days] of age): Weight-based dosing of the oral suspension as specified in Table 5.
- No data are available in pre-term neonates. The use of ISENTRESS is not recommended in pre-term neonates.
|
Body Weight |
Volume (Dose) of Suspension to be Administered |
|---|---|
|
Note: If the mother has taken ISENTRESS or ISENTRESS HD 2-24 hours before delivery, the neonate's first dose should be given between 24-48 hours after birth. | |
| |
|
Birth to 1 Week - Once daily dosing***** | |
|
2 to less than 3 |
0.4 mL (4 mg) once daily |
|
3 to less than 4 |
0.5 mL (5 mg) once daily |
|
4 to less than 5 |
0.7 mL (7 mg) once daily |
|
1 to 4 Weeks - Twice daily dosing**†** | |
|
2 to less than 3 |
0.8 mL (8 mg) twice daily |
|
3 to less than 4 |
1 mL (10 mg) twice daily |
|
4 to less than 5 |
1.5 mL (15 mg) twice daily |
ISENTRESS and ISENTRESS HD can be administered with or without food (2.1).
Do not substitute ISENTRESS chewable tablets or ISENTRESS for oral suspension for the ISENTRESS 400 mg or 600 mg film-coated tablet.
See specific dosing guidance for chewable tablets and the formulation for oral suspension (2.1).
Adults
- Treatment-naïve patients or patients who are virologically suppressed on an initial regimen of ISENTRESS 400 mg twice daily:
- 1200 mg (2 × 600 mg) film-coated tablet orally, once daily or
- 400 mg film-coated tablet orally, twice daily (2.2).
- Treatment-experienced patients:
- 400 mg film-coated tablet orally, twice daily (2.2).
- During coadministration with rifampin in adults, 800 mg (2 × 400 mg) twice daily (2.2).
Pediatrics
- If weighing at least 40 kg, and either
- treatment-naïve patients or
- patients who are virologically suppressed on an initial regimen of ISENTRESS 400 mg twice daily:
- 1200 mg (2 × 600 mg) film-coated tablet orally, once daily or
- 400 mg film-coated tablet orally, twice daily or
- 300 mg (3 × 100 mg) chewable tablets, twice daily (2.3).
- If weighing at least 25 kg: One 400 mg film-coated tablet orally, twice daily. If unable to swallow a tablet, consider the chewable tablet, as specified in Table 2 (2.3).
- If weighing at least 3 kg to less than 25 kg: Weight-based dosing using the chewable tablet or oral suspension, as specified in Table 4 (2.3).
- For neonates (birth to 4 weeks [28 days] of age): Weight-based dosing of the oral suspension as specified in Table 5 (2.3).