FEELSHION Lavender Magnesium
3513964e-dc5a-ab1e-e063-6294a90a993d
HUMAN OTC DRUG LABEL
May 14, 2025
Beijing JUNGE Technology Co., Ltd.
DUNS: 848718652
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lavender Magnesium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (22)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
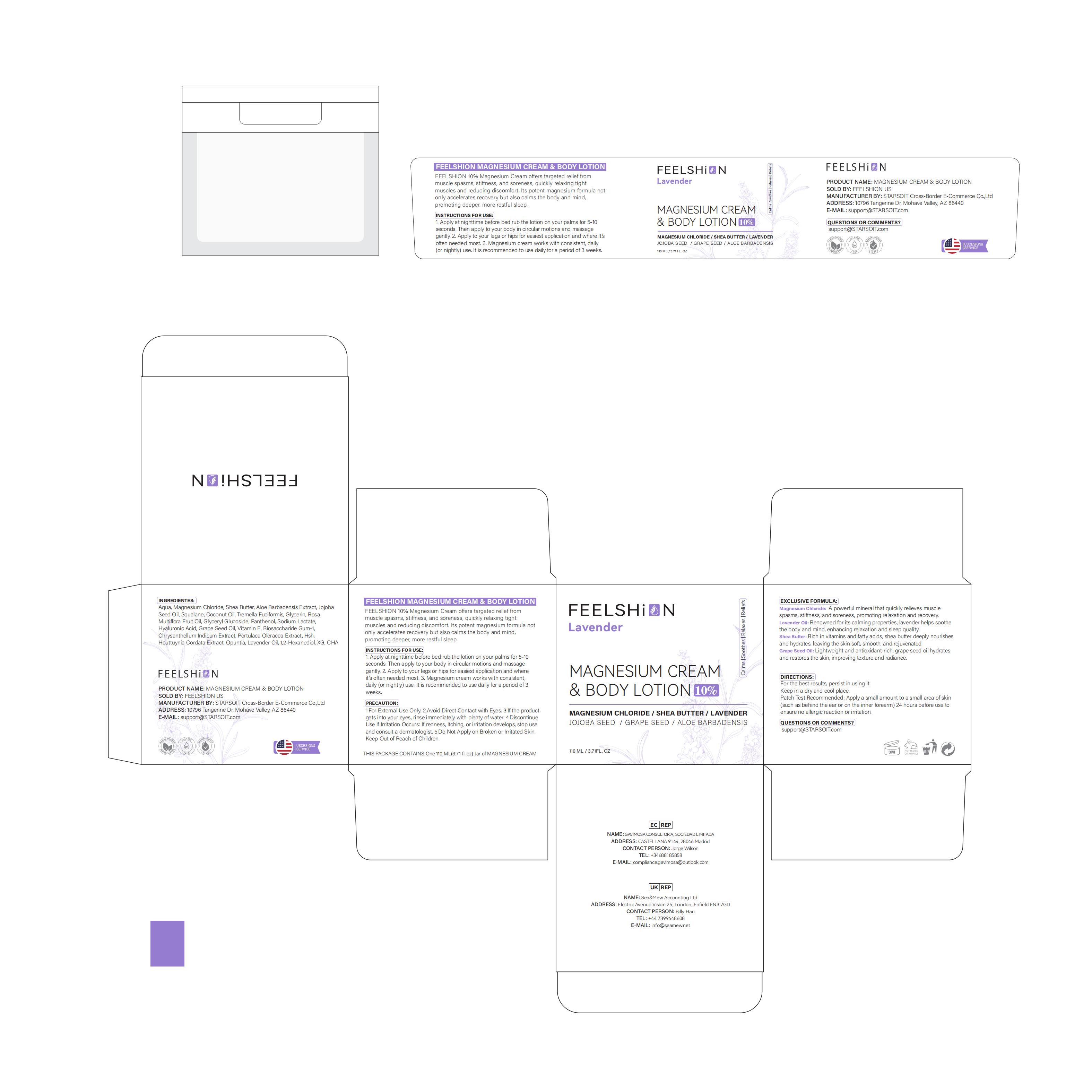
INDICATIONS & USAGE SECTION
Skin
INACTIVE INGREDIENT SECTION
AQUA
BUTYROSPERMUM PARKII (SHEA BUTTER)
ALOE BARBADENSIS FLOWER EXTRACT
SQUALANE
COCOS NUCIFERA (COCONUT) OIL
TREMELLA FUCIFORMIS POLYSACCHARIDE
GLYCERIN
ROSA MULTIFLORA FRUIT EXTRACT
PANTHENOL
SODIUM LACTATE
BIOSACCHARIDE GUM-1
CHRYSANTHELLUM INDICUM EXTRACT
PORTULACA OLERACEA EXTRACT
HOUTTUYNIA CORDATA EXTRACT
OPUNTIA TUNA FRUIT EXTRACT
1,2-HEXANEDIOL
DOSAGE & ADMINISTRATION SECTION
Apply an appropriate amount of magnesium cream to the desired location.
WARNINGS SECTION
For external use only.
OTC - STOP USE SECTION
rash occurs.
OTC - DO NOT USE SECTION
on damaged or broken skin.
OTC - WHEN USING SECTION
Please use this product before going to bed, apply thisproduct to the neck, shoulders, legs, etc, Gentlymassage for 5-10 seconds until completely absorbed.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
If swallowed, get medical help or contact a Poison Control Center right away.
OTC - PURPOSE SECTION
*Helps solve sleep problems
*Promote deep relaxation
Relieve muscle tensionlmprove sleep quality
OTC - ACTIVE INGREDIENT SECTION
MAGNESIUM CHLORIDE,SIMMONDSIA CHINENSIS (JOJOBA) SEED OIL,HYALURONIC ACID,VITIS VINIFERA (GRAPE) SEED OIL,TOCOPHEROL,LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL
