Stomachin Antacid
Stomachin
60fa0650-8785-4cb3-8f88-1a437296252f
HUMAN OTC DRUG LABEL
Sep 19, 2025
Chang Kuo Chou Pharmaceutical Co., Ltd.
DUNS: 656241486
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
magnesium carbonate and sodium bicarbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
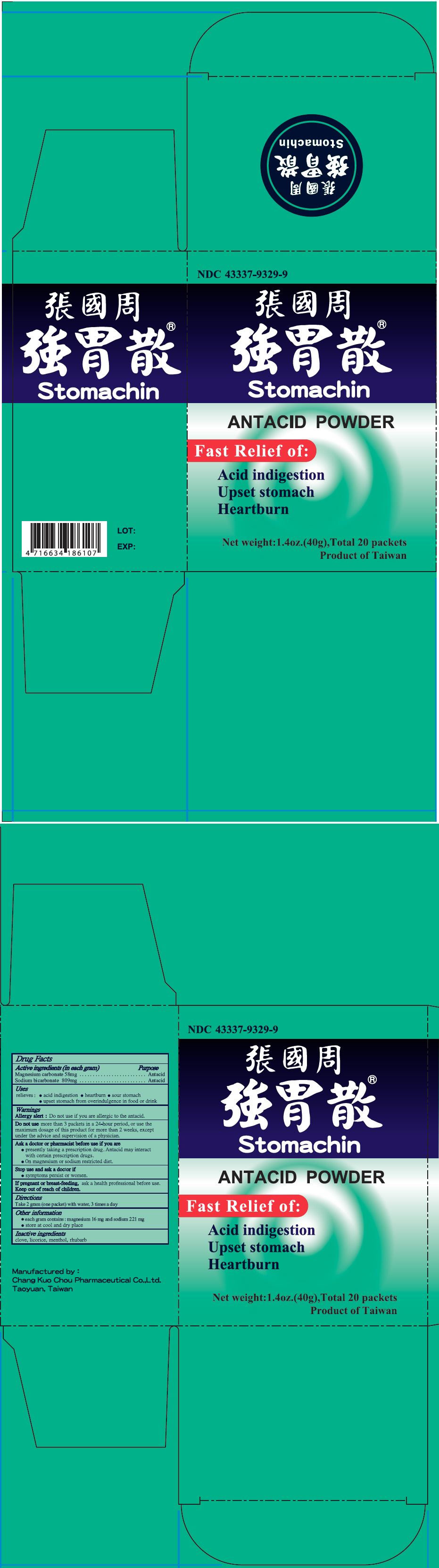
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 40 g Packet Box
NDC 43337-9329-9
Stomachin ®
ANTACID POWDER
Fast Relief of:
Acid indigestion
Upset stomach
Heartburn
Net weight: 1.4oz.(40g), Total 20 packets
Product of Taiwan
INDICATIONS & USAGE SECTION
Uses
relieves:
- acid indigestion
- heartburn
- sour stomach
- upset stomach from overindulgence in food or drink
SPL UNCLASSIFIED SECTION
Manufactured by :
** Chang Kuo Chou Pharmaceutical Co.,Ltd.**
** Taoyuan, Taiwan**
OTC - ACTIVE INGREDIENT SECTION
|
Active ingredients (in each gram) |
Purpose |
|---|---|
|
Magnesium carbonate 58mg |
Antacid |
|
Sodium bicarbonate 809mg |
Antacid |
WARNINGS SECTION
Warnings
Allergy alert
Do not use if you are allergic to the antacid.
Do not usemore than 3 packets in a 24-hour period, or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a physician.
Ask a doctor or pharmacist before use if you are
- presently taking a prescription drug. Antacid may interact with certain prescription drugs.
- On magnesium or sodium restricted diet.
Stop use and ask a doctor if
- symptoms persist or worsen.
**If pregnant or breast-feeding,**ask a health professional before use.
Keep out of reach of children.
DOSAGE & ADMINISTRATION SECTION
Directions
Take 2 gram (one packet) with water, 3 times a day
STORAGE AND HANDLING SECTION
Other information
- each gram contains : magnesium 16 mg and sodium 221 mg
- store at cool and dry place
INACTIVE INGREDIENT SECTION
Inactive ingredients
clove, licorice, menthol, rhubarb
