diltiazem hydrochloride
Diltiazem HCl Extended-Release Capsules, USPFor Oral Administration
ec928ee5-0809-4ba5-82ca-ecfed99ebb5a
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
diltiazem hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (24)
Drug Labeling Information
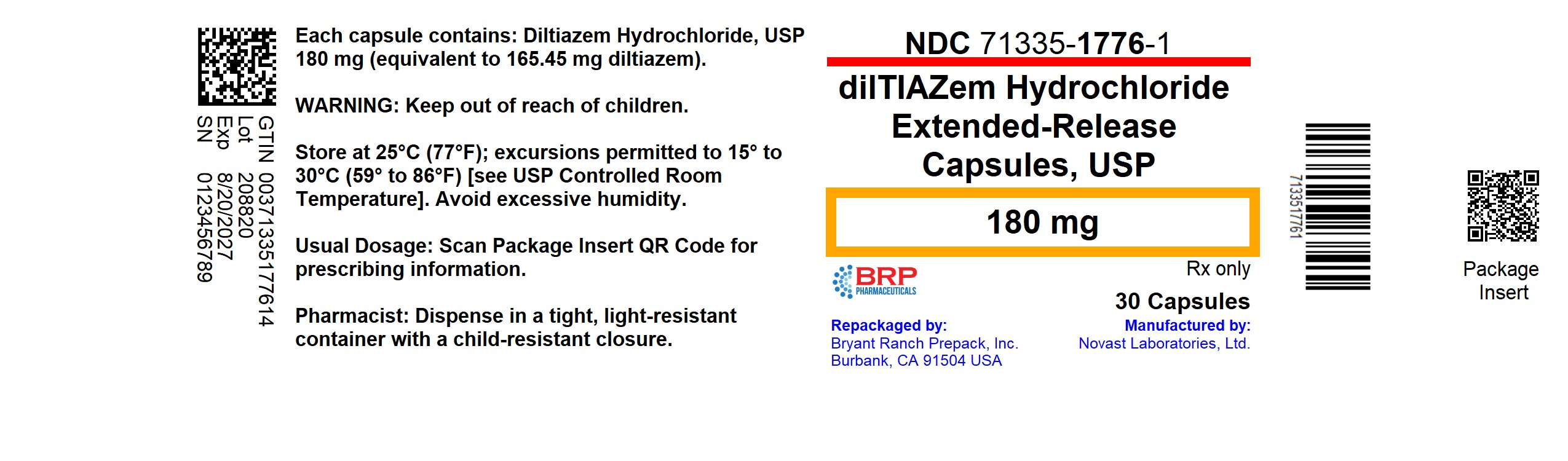
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Diltiazem Hcl ER 180mg Capsule
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Serious adverse reactions have been rare in studies carried out to date, but it should be recognized that patients with impaired ventricular function and cardiac conduction abnormalities have usually been excluded from these studies.
The following table presents the most common adverse reactions reported in placebo-controlled angina and hypertension trials in patients receiving diltiazem hydrochloride extended-release capsules up to 360 mg with rates in placebo patients shown for comparison.
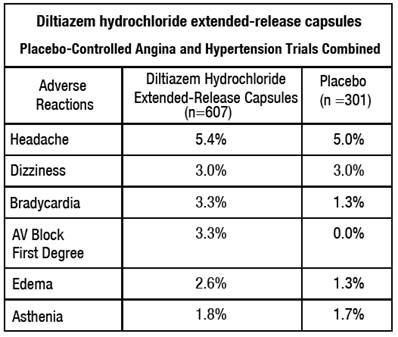
In addition, the following events were reported infrequently (less than 1%) in angina or hypertension trials:
**Cardiovascular:**Congestive heart failure, palpitations, syncope, ventricular extrasystoles.
**Nervous System:**Abnormal dreams, amnesia, depression, gait abnormality, hallucinations, insomnia, nervousness, paresthesia, personality change, somnolence, tinnitus, tremor.
G****astrointestinal:Anorexia, constipation, diarrhea, dry mouth, dysgeusia, dyspepsia, mild elevations of SGOT, SGPT, LDH, and alkaline phosphatase (seeWARNINGS, Acute Hepatic Injury), thirst, vomiting, weight increase.
**Dermatological:**Petechiae, photosensitivity, pruritus, urticaria.
**Other:**Amblyopia, CPK increase, dyspnea, epistaxis, eye irritation, hyperglycemia, hyperuricemia, impotence, muscle cramps, nasal congestion, nocturia, osteoarticular pain, polyuria, sexual difficulties.
The following postmarketing events have been reported infrequently in patients receiving diltiazem hydrochloride: acute generalized exanthematous pustulosis, allergic reactions, alopecia, angioedema (including facial or periorbital edema), asystole, erythema multiforme (including Stevens-Johnson syndrome, toxic epidermal necrolysis), exfoliative dermatitis, extrapyramidal symptoms, gingival hyperplasia, hemolytic anemia, increased bleeding time, leukopenia, photosensitivity (including lichenoid keratosis and hyperpigmentation at sun- exposed skin areas), purpura, retinopathy, myopathy, and thrombocytopenia. In addition, events such as myocardial infarction have been observed which are not readily distinguishable from the natural history of the disease in these patients. A number of well-documented cases of generalized rash, some characterized as leukocytoclastic vasculitis, have been reported. However, a definitive cause and effect relationship between these events and diltiazem hydrochloride therapy is yet to be established.
To report SUSPECTED ADVERSE REACTIONS, contact Ingenus Pharmaceuticals,LLC Toll-Free at 1-877-748-1970 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch**.**
DESCRIPTION SECTION
DESCRIPTION
Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1, 5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2, 3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride,(+)-cis-. The chemical structure is:
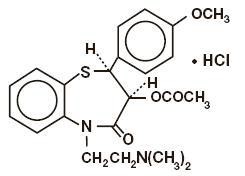
USP Dissolution pending.
Diltiazem hydrochloride, USP is a white to off-white crystalline powder with a bitter taste. It is soluble in water, methanol, and chloroform. It has a molecular weight of 450.98. Diltiazem hydrochloride extended-release capsule, USP is formulated as a once-a-day extended-release capsule containing 120 mg, 180 mg, 240 mg, 300 mg, or 360 mg diltiazem hydrochloride.
Capsules also contain: hypromellose, sucrose, starch (maize), methacrylic acid and ethyl acrylate copolymer, triethyl citrate, talc, hydroxypropylcellulose, ammonio methacrylate copolymer, ethylcellulose, diethyl phthalate, magnesium stearate, titanium dioxide, polydextrose, triacetin, Macrogol/PEG, gelatin, sodium lauryl sulphate, shellac, potassium hydroxide, black iron oxide, FD&C Blue #1(180 mg, 240 mg, 300 mg and 360 mg) and FD&C Yellow#6 (180 mg and 240 mg).
HOW SUPPLIED SECTION
HOW SUPPLIED
Diltiazem hydrochloride Extended-Release Capsules, USP
|
Strength |
Quantity |
NDC Number |
Description |
Capsule |
|
180 mg |
30 Capsules in a Bottle 90 Capsules in a Bottle |
71335-1776-1 71335-1776-2 |
Light blue/light blue capsule (size 1) imprinted with N366 on one end and 180 on the otherend. |
|
Storage Conditions: Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Avoid
excessive humidity.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504

