Simulect
Simulect (basiliximab) For Injection Rx only Prescribing Information
1af01887-b69d-444b-91ed-ebfe12784440
HUMAN PRESCRIPTION DRUG LABEL
Aug 1, 2023
Novartis Pharmaceuticals Corporation
DUNS: 002147023
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
basiliximab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
basiliximab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
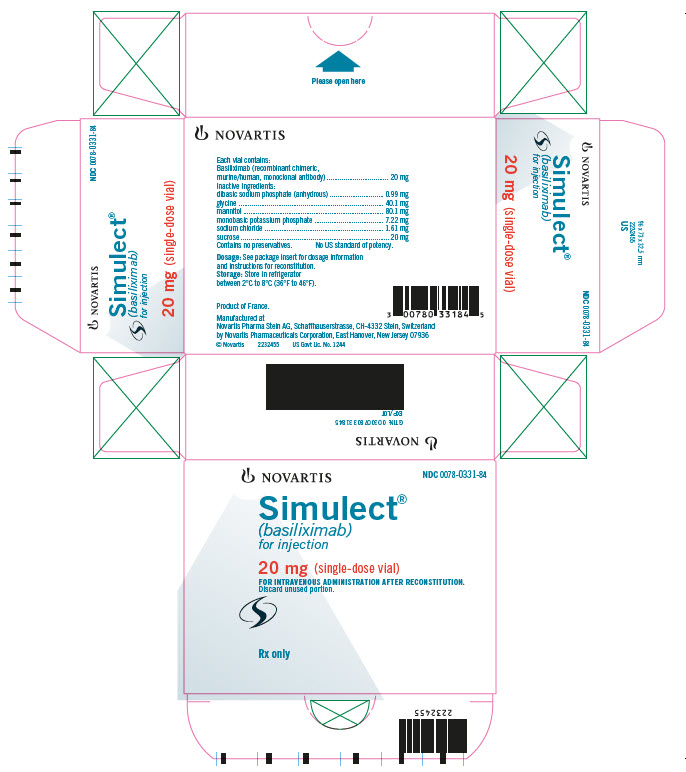
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NOVARTIS NDC 0078-0331-84
Simulect®
(basiliximab)
for injection
20 mg (single-dose vial)
FOR INTRAVENOUS ADMINISTRATION AFTER RECONSTITUTION.
Discard unused portion.
Rx only