Phenazopyridine Hydrochloride
PHENAZOPYRIDINE HYDROCHLORIDE TABLETS, USP Rx only
a7be39db-8eb8-9cc6-e053-2a95a90a8930
HUMAN PRESCRIPTION DRUG LABEL
Feb 7, 2022
NuCare Pharmaceuticals,Inc.
DUNS: 010632300
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Phenazopyridine Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label

INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Phenazopyridine HCL is indicated for the symptomatic relief of pain, burning, urgency frequency, and other discomforts arising from irritation of the mucosa of the lower urinary tract caused by infection, trauma, surgery, endoscopic procedures, or the passage of sounds or catheters.
The use of Phenazopyridine for relief of symptoms should not delay definitive diagnosis and treatment of causative conditions. The drug should be used for symptomatic relief of pain and not as a substitute for specific surgery or antimicrobial therapy.
Phenazopyridine is compatible with antimicrobial therapy and can help relieve pain and discomfort during the interval before antimicrobial therapy controls the infection.
Treatment of a urinary tract infection with Phenazopyridine should not exceed 2 days. There is no evidence that the combined administration of Phenazopyridine and an antimicrobial provides greater benefit than administration of the antimicrobial alone after 2 days. (See Dosage and Administration.)
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
In patients who are hypersensitive to the drug or its ingredients. Phenazopyridine is contraindicated in patients with renal insufficiency, severe liver disease, severe hepatitis or pyelonephritis of pregnancy.
It should be used cautiously in the presence of GI disturbances.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
The following adverse events have been reported:
CNS: headache.
Gastrointestinal: nausea, vomiting and diarrhea.
Dermatologic and Hypersensitivity: rash, pruritus, discoloration, anaphylactoid-like reaction and hypersensitivity hepatitis.
Hematologic: methemoglobinemia, hemolytic anemia, potential hemolytic agent in G-6-PD deficiency, sulfhemoglobinemia.
Other: visual disturbances, renal and hepatic toxicity usually associated with overdose, renal calculi, jaundice, discoloration of body fluids and aseptic meningitis.
SPL UNCLASSIFIED SECTION
Manufactured for:
Westminster Pharmaceuticals, LLC
154 Downing Street, Unit 1 & 2
Olive Branch, MS 38654
Rev: 10/18
DESCRIPTION SECTION
DESCRIPTION
Phenazopyridine Hydrochloride is a reddish-brown, odorless, slightly bitter, crystalline powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain.
Following is the structural formula:
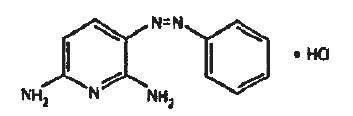
Phenazopyridine HCl oral tablets contain the following inactive ingredients: Crospovidone, Macrogol, Polysorbate 80, Polyvinyl Alcohol, Talc.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Phenazopyridine hydrochloride is excreted in the urine where it exerts a topical analgesic effect on the mucosa of the urinary tract. This action helps to relieve pain, burning, urgency and frequency. The precise mechanism of action is unknown.
PHARMACOKINETICS
The pharmacokinetic properties of Phenazopyridine hydrochloride have not been determined. Phenazopyridine and its metabolites are rapidly excreted by the kidneys. In a small number of healthy subjects, 90% of a 600 mg/day oral dose of Phenazopyridine hydrochloride was eliminated in the urine in 24 hours, 41% as unchanged drug and 49% as metabolites.
WARNINGS SECTION
WARNINGS
Phenazopyridine hydrochloride is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals (IARC 1980, 1982, 1987, NCI 1978). When administered in the diet, Phenazopyridine hydrochloride increased the incidences of hepatocellular adenomas and carcinomas in female mice and adenomas and adenocarcinomas of the colon and rectum in rats of both sexes. There is inadequate evidence for the carcinogenicity of Phenazopyridine hydrochloride in humans (TARC 1987). In one limited epidemiological study, no significant excess of any cancer was observed among 2,214 patients who received Phenazopyridine hydrochloride and were followed for a minimum of 3 years.
PRECAUTIONS SECTION
PRECAUTIONS
General
The patient should be advised that Phenazopyridine produces an orange to red color in the urine and feces, and may cause staining. Phenazopyridine may cause discoloration of body fluids and staining of contact lenses has been reported. A yellowish color of the skin or sclera may indicate accumulation of Phenazopyridine resulting from impaired renal function and necessitates discontinuance of the drug. It should be noted that a decline in renal function is common in elderly patients. Phenazopyridine may mask pathological conditions and interfere with laboratory test values using colorimetric, spectrophotometric or fluorometric analysis methods.
Cautious use in patients with G-6-PD deficiency is advised since these patients are susceptible to oxidative hemolysis and may have greater potential to develop hemolytic anemia.
Information for Patients
The patient should be advised to take Phenazopyridine with or following food or after eating a snack to reduce stomach upset.
The patients should be aware that Phenazopyridine causes a reddish orange discoloration of the urine and feces, and may stain clothing. Phenazopyridine may cause discoloration of body fluids and staining of contact lenses has been reported. There have been reports of teeth discoloration when the product has been broken or held in the mouth prior to swallowing.
Patients should be instructed to take Phenazopyridine for only 2 days if an antibacterial agent is administered concurrently for the treatment of a urinary tract infection. If symptoms persist beyond those 2 days, the patient should be instructed to contact his or her physician.
Laboratory Tests
Phenazopyridine may interfere with laboratory test values using colorimetric, photometric or fluorometric analysis methods.
Altered urine laboratory test values may include ketone (sodium nitroprusside) bilirubin (foam test, talc-disk-Fouchet-spot test, Franklin's tablet-Fouchet test, p-nitrobenzene diazonium p-toluene sulfonate reagent), diacetic acid (Gerhardt ferric chloride test), free hydrochloric acid, glucose (glucose oxidase tests), 17-hydroxycorticosteroids (modified Glenn-Nelson), 17-ketosteroids (Holtorff Koch modification of Zimmerman), porphyrins, albumin (discolors bromophenol blue test areas of commercial reagent strips, nitric acid ring test), phenolsulfophthalein, urobilinogen (color interference with Ehrlich 's reagent), and urinalysis (spectrophotometric or color-based tests). Phenazopyridine also imparts an orange-red color to stools which may interfere with color tests.
Drug Interactions
The interaction of Phenazopyridine with other drugs has not been studied in a systematic manner. However, the medical literature to date suggests that no significant interactions have been reported.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term administration of Phenazopyridine has been associated with tumors of the large intestine in rats and of the liver in mice. Available epidemiological data are insufficient to evaluate the carcinogenicity of Phenazopyridine in humans. In vitro studies indicate that Phenazopyridine in the presence of metabolic activation is mutagenic in bacteria and mutagenic and clastogenic in mammalian cells.
Pregnancy Category B
Reproductive studies with Phenazopyridine (in combination with sulfacytine) in rats given up to 110 mg/kg/day and in rabbits given up to 39 mg/kg/day during organogenesis revealed no evidence of harm to offspring.
One prospective study in humans demonstrated that Phenazopyridine traverses the placenta into the fetal compartment. There are no adequate and well- controlled studies in pregnant women. Therefore, Phenazopyridine should be used in pregnant women only if the benefit clearly outweighs the risk.
Nursing Mothers
It is not known whether Phenazopyridine or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of drug therapy to the mother.
Children
Adequate and well-controlled studies have not been performed in the pediatric population. No pediatric-specific problems have been documented.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
100 mg Tablets: Average adult dosage is two tablets 3 times a day after meals.
200 mg Tablets: Average adult dosage is one tablet 3 times a day after meals.
When used concomitantly with an antibacterial agent for the treatment of a urinary tract infection, the administration of Phenazopyridine Hydrochloride should not exceed 2 days.
HOW SUPPLIED SECTION
HOW SUPPLIED
Dispense contents with a child-resistant closure (as required) and in a tight container as defined in the USP.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature].
NDC 69367-162-04 100 mg, 100 count - Appearance: Reddish-brown, round, film coated tablets debossed 812 on one side and plain on the other.
NDC 68071-5274-1 BOTTLES OF 10
To report an adverse reaction, please contact Westminster Pharmaceuticals, LLC at 1-844-221-7294
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
