MYXREDLIN
These highlights do not include all the information needed to use safely and effectively. See full prescribing information for . Initial U.S. Approval: 2019
afca6e80-a802-49fb-a978-5fe28a173001
HUMAN PRESCRIPTION DRUG LABEL
Jun 4, 2020
Baxter Healthcare Corporation
DUNS: 005083209
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
insulin human
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
****
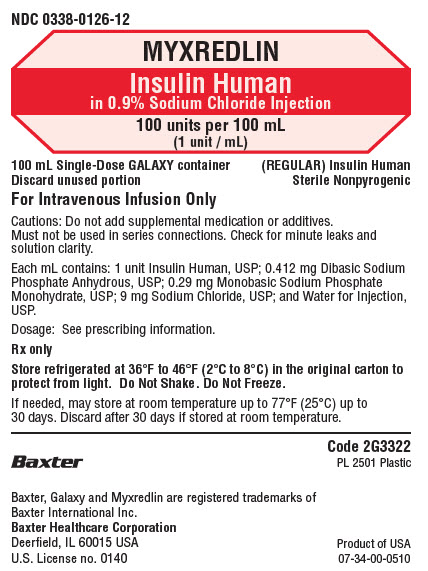
NDC 0338-0126-12
MYXREDLIN
Insulin Human
in 0.9% Sodium Chloride Injection
100 units per 100 mL
(1 unit / mL)
100 mL Single-Dose GALAXY container
Discard unused portion
For Intravenous Infusion Only
(REGULAR) Insulin Human
Sterile Nonpyrogenic
Cautions: Do not add supplemental medication or additives.
Must not be used in series connections. Check for minute leaks and
solution clarity.
Each mL contains: 1 unit Insulin Human, USP; 0.412 mg Dibasic Sodium
Phosphate Anhydrous, USP; 0.29 mg Monobasic Sodium Phosphate
Monohydrate, USP; 9 mg Sodium Chloride, USP; and Water for Injection,
USP.
Dosage: See prescribing information.
Rx only
Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to
protect from light. Do Not Shake. Do Not Freeze.
If needed, may store at room temperature up to 77°F (25°C) up to
30 days. Discard after 30 days if stored at room temperature.
Baxter logo
Baxter, Galaxy and Myxredlin are trademarks of
Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
U.S. License no. 0140
Code 2G3322
PL 2501 Plastic
Product of USA
07-34-00-0510

NDC 0338-0126-12
Code 2G3322
MYXREDLIN
Insulin Human
in 0.9% Sodium Chloride Injection
100 units per 100 mL
(1 unit / mL)
For Intravenous Infusion Only
(REGULAR) Insulin Human
USE CARTON TO PROTECT CONTENTS
FROM LIGHT WHILE REFRIGERATED
Rx Only
1 Single-Dose Galaxy container
Discard unused portion
Baxter Logo
For Intravenous Infusion Only
MYXREDLIN
Insulin Human
in 0.9% Sodium Chloride Injection
CONTAINS INSULIN HUMAN
100 units per 100 mL
(1 unit/mL)
NDC 0338-0126-12
MYXREDLIN
Insulin Human
in 0.9% Sodium Chloride Injection
****(1 unit/mL)
UNVARNISHED AREA FOR ON-LINE
PRINTING OF LOT AND EXP
UPC-A
Bar Code
Placement
Sterile, Nonpyrogenic
Each mL contains: 1 unit Insulin Human, USP; 0.412 mg Dibasic Sodium Phosphate Anhydrous,
USP; 0.29 mg Monobasic Sodium Phosphate Monohydrate, USP; 9 mg Sodium Chloride, USP;
and Water for Injection, USP. No preservative.
**Dosage:**For Intravenous Infusion Only. See prescribing information.
**Caution:**Do not add supplemental medication or additives.
Rx only
Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
Do Not Shake. Do Not Freeze.
If needed, may store MYXREDLIN at room temperature up to 77°F (25°C) up to 30
days.
Once stored at room temperature, do not place back in the refrigerator.
Discard after 30 days if stored at room temperature.
Discard 30 days after storing at room temperature. Discard After:
(Month) (Day) (Year)
(to be completed by dispensing pharmacist)
Baxter, Galaxy and Myxredlin are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
U.S. License no. 0140
Product of USA
07-01-00-0251
G
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Table 1: Clinically Significant Drug Interactions with MYXREDLIN|
Drugs that May Increase the Risk of Hypoglycemia | |
|
Drugs: |
Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics. |
|
Intervention: |
Dose adjustment and increased frequency of glucose monitoring may be required when MYXREDLIN is co-administered with these drugs. |
|
Drugs that May Decrease the Blood Glucose Lowering Effect of MYXREDLIN | |
|
Drugs: |
Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. |
|
Intervention: |
Dose adjustment and increased frequency of glucose monitoring may be required when MYXREDLIN is co-administered with these drugs. |
|
Drugs that May Increase or Decrease the Blood Glucose Lowering Effect of MYXREDLIN | |
|
Drugs: |
Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. |
|
Intervention: |
Dose adjustment and increased frequency of glucose monitoring may be required when MYXREDLIN is co-administered with these drugs. |
|
Drugs that May Blunt Signs and Symptoms of Hypoglycemia | |
|
Drugs: |
Beta-blockers, clonidine, guanethidine, and reserpine. |
|
Intervention: |
Increased frequency of glucose monitoring may be required when MYXREDLIN is co-administered with these drugs. |
•
Drugs that may increase the risk of hypoglycemia: antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics. (7)
•
Drugs that may decrease the blood glucose lowering effect: atypical antipsychotics, corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. (7)
•
Drugs that may increase or decrease the blood glucose lowering effect: Alcohol, beta-blockers, clonidine, lithium salts, and pentamidine. (7)
•
Drugs that may blunt the signs and symptoms of hypoglycemia: beta-blockers, clonidine, guanethidine, and reserpine. (7)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard 2-year carcinogenicity studies in animals have not been performed to evaluate the carcinogenic potential of insulin human injection.
Human insulin is not mutagenic in the following in vitro tests: The chromosomal aberration assay in human lymphocytes, the micronucleus assay in mouse polychromatic erythrocytes, and the mutation frequency assay in Chinese hamster cells.
Standard reproduction and teratology studies in animals, including fertility assessments have not been conducted with insulin human injection.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
MYXREDLIN (insulin human) in 0.9% sodium chloride injection contains 100 units/100 mL (1 unit/mL) of insulin human in 0.9% sodium chloride and is a clear, colorless solution available as:
100 mL single-dose GALAXY container, package of 12, NDC 0338-0126-12
16.2 Storage and Handling
Store MYXREDLIN in the refrigerator (36°F to 46°F [2°C to 8°C]) in the original carton to protect from light. Do not use after the expiration date printed on the carton and container label.
If needed, MYXREDLIN may be removed from the original carton and stored at room temperature up to 77°F (25°C) for up to 30 days. Once stored at room temperature, do not place back in the refrigerator. Discard MYXREDLIN after 30 days if stored at room temperature.
Do not freeze and do not use MYXREDLIN if it has been frozen. Do not shake.
