Warfarin Sodium
These highlights do not include all the information needed to use WARFARIN SODIUM TABLETS safely and effectively. See full prescribing information for WARFARIN SODIUM TABLETS. WARFARIN SODIUM tablets, for oral useInitial U.S. Approval: 1954
d166f83b-7052-4dc7-b7d4-47faae7dcaed
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
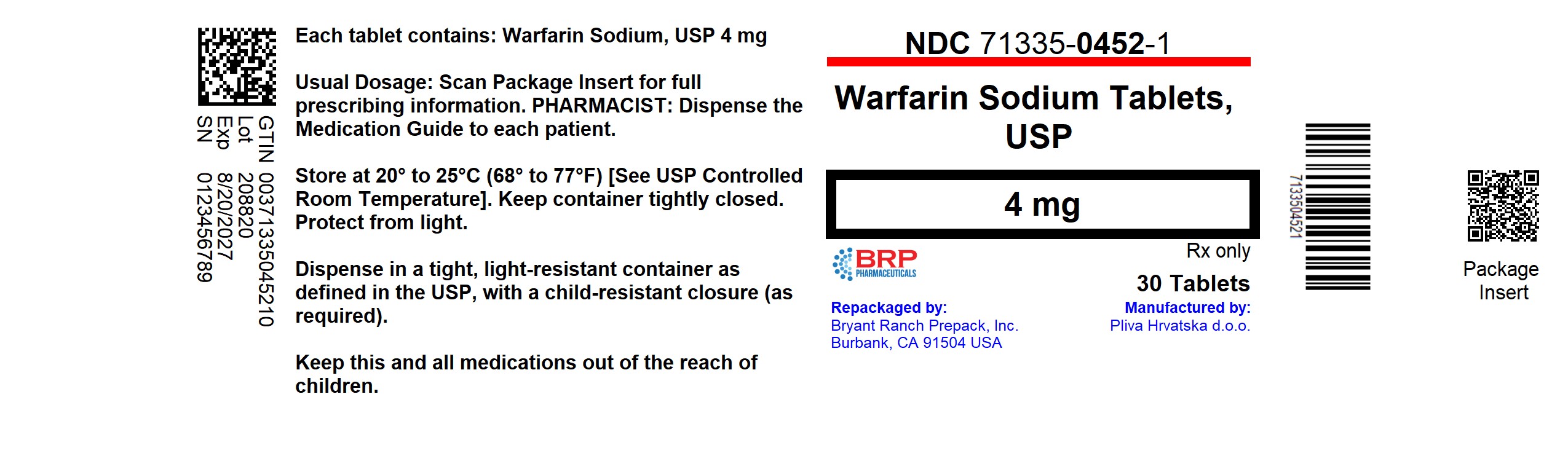
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Warfarin Sodium 4mg Tablet
DESCRIPTION SECTION
11 DESCRIPTION
Warfarin sodium tablets, USP contain warfarin sodium, an anticoagulant that acts by inhibiting vitamin K-dependent coagulation factors. The chemical name of warfarin sodium is 3-(α-acetonylbenzyl)-4-hydroxycoumarin sodium salt, which is a racemic mixture of the R- and S-enantiomers. Crystalline warfarin sodium is an isopropanol clathrate. Its structural formula may be represented as follows:
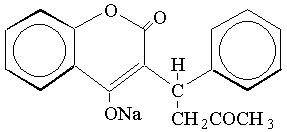
C19H15NaO4 M.W. 330.31
Crystalline warfarin sodium occurs as a white, odorless, crystalline powder that is discolored by light. It is very soluble in water, freely soluble in alcohol, and very slightly soluble in chloroform and ether.
Each tablet, for oral administration, contains 1 mg, 2 mg, 2.5 mg, 3 mg, 4 mg, 5 mg, 6 mg, 7.5 mg or 10 mg warfarin sodium, USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose.
The 1 mg also contains FD&C red no. 40.
The 2 mg also contains FD&C blue no. 2 aluminum lake and FD&C red no. 40 aluminum lake.
The 2.5 mg also contains D&C yellow no. 10 aluminum lake and FD&C blue no. 1 aluminum lake.
The 3 mg also contains FD&C yellow no. 6 aluminum lake, FD&C blue no. 2 aluminum lake, FD&C red no. 40 aluminum lake, and D&C yellow no. 10 aluminum lake.
The 4 mg also contains FD&C blue no. 1 aluminum lake and FD&C blue no. 2 aluminum lake.
The 5 mg also contains FD&C yellow no. 6 aluminum lake, FD&C red no. 40 aluminum lake and D&C yellow no. 10 aluminum lake.
The 6 mg also contains D&C yellow no. 10 aluminum lake and FD&C blue no. 1 aluminum lake.
The 7.5 mg also contains D&C yellow no. 10 aluminum lake and FD&C yellow no. 6 aluminum lake.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Warfarin sodium tablets USP, 4 mg are available as blue, capsule-shaped, biconvex scored tablets, debossed with TV/4 on the scored side and 1716 on the other side containing 4 mg warfarin sodium, USP,
NDC: 71335-0452-1: 30 Tablets in a BOTTLE
NDC: 71335-0452-2: 60 Tablets in a BOTTLE
NDC: 71335-0452-3: 90 Tablets in a BOTTLE
NDC: 71335-0452-4: 100 Tablets in a BOTTLE
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Protect from light. Keep tightly closed.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Special Handling
Procedures for proper handling and disposal of potentially hazardous drugs should be considered. Guidelines on this subject have been published [see References (15)].
Pharmacy and clinical personnel who are pregnant should avoid exposure to crushed or broken tablets [see Use in Specific Populations (8.1)].
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Instructions for Patients
Advise patients to:
- Strictly adhere to the prescribed dosage schedule [see Dosage and Administration (2.1)].
- If the prescribed dose of warfarin sodium is missed, take the dose as soon as possible on the same day but do not take a double dose of warfarin sodium the next day to make up for missed doses [see Dosage and Administration (2.6)].
- Obtain prothrombin time tests and make regular visits to their physician or clinic to monitor therapy [see Dosage and Administration (2.1)].
- Be aware that if therapy with warfarin sodium is discontinued, the anticoagulant effects of warfarin sodium may persist for about 2 to 5 days [see Clinical Pharmacology (12.2)].
- Avoid any activity or sport that may result in traumatic injury [see Use in Specific Populations (8.4)]. And to tell their physician if they fall often as this may increase their risk for complications.
- Eat a normal, balanced diet to maintain a consistent intake of vitamin K. Avoid drastic changes in dietary habits, such as eating large amounts of leafy, green vegetables [see Drug Interactions (7.5)].
- Contact their physician to report any serious illness, such as severe diarrhea, infection, or fever [see Warnings and Precautions (5) and Adverse Reactions (6)].
- Immediately contact their physician when experiencing pain and discoloration of the skin (a purple bruise like rash) mostly on areas of the body with a high fat content, such as breasts, thighs, buttocks, hips and abdomen [see Warnings and Precautions (5.2)].
- Immediately contact their physician when experiencing any unusual symptom or pain since warfarin sodium may cause small cholesterol or athero emboli. On feet it may appear as a sudden cool, painful, purple discoloration of toe(s) or forefoot [see Warnings and Precautions (5.5)].
- Immediately contact their physician when taking warfarin sodium after any heparin formulation therapy and experiencing bloody or black stools or appearence of bruises, or bleeding [see Warnings and Precautions (5.6)].
- To tell all of their healthcare professionals and dentists that they are taking warfarin sodium. This should be done before they have any surgery or dental procedure [see Dosage and Administration (2.7)].
- Carry identification stating that they are taking warfarin sodium.
Bleeding Risks
Advise patients to:
- Notify their physician immediately if any unusual bleeding or symptoms occur. Signs and symptoms of bleeding include: pain, swelling or discomfort, prolonged bleeding from cuts, increased menstrual flow or vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or dark brown urine, red or tar black stools, headache, dizziness, or weakness [see Box Warning and Warnings and Precautions (5.1)].
Concomitant Medications and Botanicals (Herbals)
Advise patients to:
- Not take or discontinue any other drug, including salicylates (e.g., aspirin and topical analgesics), other over-the-counter drugs, and botanical (herbal) products except on advice of your physician [see Drug Interactions (7)].
Pregnancy and Nursing
Advise patients to:
- Notify their physician if they are pregnant or planning to become pregnant or considering breast feeding [see Use in Specific Populations (8.1, 8.2, 8.3)].
- Avoid warfarin sodium during pregnancy except in pregnant women with mechanical heart valves, who are at risk of thromboembolism [see Contraindications (4)]. Use effective measures to avoid pregnancy while taking warfarin sodium. This is very important because their unborn baby could be seriously harmed if they take warfarin sodium while they are pregnant [see Use in Specific Populations (8.1, 8.3)].
Dispense with Medication Guide available at: www.tevausa.com/medguides
Manufactured In Croatia By:
**Pliva Hrvatska d.o.o.**Zagreb, Croatia
Manufactured For:
Teva PharmaceuticalsParsippany, NJ 07054
Rev. F 8/2021
