Ipratropium Bromide
PRESCRIBING INFORMATION IPRATROPIUM BROMIDE NASAL SOLUTION, 0.03% Nasal Spray Rx Only
6493c00f-2306-2cb6-9624-4ea678f66576
HUMAN PRESCRIPTION DRUG LABEL
Sep 18, 2025
NORTHSTAR RX LLC
DUNS: 830546433
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ipratropium Bromide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 0.03 percent Carton Label
Northstar Rx LLC NDC 16714-526-01
Ipratropium Bromide Nasal Solution, 0.03%
Nasal Spray
345 Metered Sprays
21 mcg/spray
Rx Only

SPL PATIENT PACKAGE INSERT SECTION
PATIENT'S INSTRUCTIONS FOR USE
Ipratropium Bromide Nasal Solution, 0.03%
Nasal Spray
(ip" ra troe' pee um broe' mide)
Rx Only
Read complete instructions carefully before using.
In order to ensure proper dosing, do not attempt to change the size of the spray opening.
Ipratropium Bromide Nasal Solution, 0.03% is indicated for the symptomatic relief of rhinorrhea (runny nose) associated with allergic and nonallergic perennial rhinitis in adults and children age 6 years and older. Ipratropium Bromide Nasal Solution, 0.03% does not relieve nasal congestion, sneezing or postnasal drip associated with allergic or nonallergic perennial rhinitis.
Read complete instructions carefully and use only as directed.
To Use:
1. Remove the clear plastic dust cap and the green safety clip from the nasal spray pump ( Figure 1). The safety clip prevents the accidental discharge of the spray in your pocket or purse.
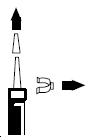
Figure 1
2. The nasal spray pump must be primed before ipratropium bromide nasal solution, 0.03% is used for the first time. To prime the pump, hold the bottle with your thumb at the base and your index and middle fingers on the white shoulder area. Make sure the bottle points upright and away from your eyes. Press your thumb firmly and quickly against the bottle seven times ( Figure 2). The pump is now primed and can be used. Your pump should not have to be reprimed unless you have not used the medication for more than 24 hours; repriming the pump will only require two sprays. If you have not used your nasal spray for more than seven days, repriming the pump will require seven sprays.
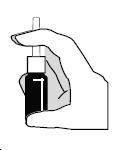
Figure 2
3. Before using ipratropium bromide nasal solution, 0.03%, blow your nose gently to clear your nostrils if necessary.
4. Close one nostril by gently placing your finger against the side of your nose, tilt your head slightly forward and, keeping the bottle upright, insert the nasal tip into the other nostril ( Figure 3). Point the tip toward the back and outer side of the nose.

Figure 3
5. Press firmly and quickly upwards with the thumb at the base while holding the white shoulder portion of the pump between your index and middle fingers. Following each spray, sniff deeply and breathe out through your mouth.
6. After spraying the nostril and removing the unit, tilt your head backwards for a few seconds to let the spray spread over the back of the nose.
7. Repeat steps 4 through 6 in the same nostril.
8. Repeat steps 4 through 7 in the other nostril (i.e., two sprays per nostril).
9. Replace the clear plastic dust cap and safety clip.
10. At some time before the medication is completely used up, you should consult your physician or pharmacist to determine whether a refill is needed. You should not take extra doses or stop using ipratropium bromide nasal solution, 0.03% without consulting your physician.
To Clean:
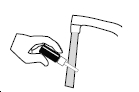
Figure 4
If the nasal tip becomes clogged, remove the clear plastic dust cap and safety clip. Hold the nasal tip under running, warm tap water ( Figure 4) for about a minute. Dry the nasal tip, reprime the nasal spray pump (step 2 above), and replace the plastic dust cap and safety clip.
Caution
Ipratropium Bromide Nasal Solution, 0.03% is intended to relieve your rhinorrhea (runny nose) with regular use. It is therefore important that you use Ipratropium Bromide Nasal Solution, 0.03% as prescribed by your physician. For most patients, some improvement in runny nose is usually apparent during the first full day of treatment with Ipratropium Bromide Nasal Solution, 0.03%. Some patients may require up to two weeks of treatment to obtain maximum benefit.
Do not spray Ipratropium Bromide Nasal Solution, 0.03% in your eyes. Should this occur, immediately flush your eye with cool tap water for several minutes. If you accidentally spray Ipratropium Bromide Nasal Solution, 0.03% in your eyes, you may experience a temporary blurring of vision, visual halos or colored images in association with red eyes from conjunctival and corneal congestion, development or worsening of narrow-angle glaucoma, pupil dilation, or acute eye pain/discomfort, and increased sensitivity to light, which may last a few hours. Should acute eye pain or blurred vision occur, contact your doctor.
Should you experience excessive nasal dryness or episodes of nasal bleeding, contact your doctor.
If you have glaucoma or difficulty urinating due to an enlargement of the prostate, be sure to tell your physician prior to using ipratropium bromide nasal solution 0.03%. .
If you are pregnant or you are breast feeding your baby, be sure to tell your physician prior to using ipratropium bromide nasal solution 0.03%.
Address medical inquiries to Northstar Rx LLC at 1-800-206-7821.
Storage
Store tightly closed at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature]. Avoid freezing. Keep out of reach of children.
|
Manufactured for: |
Manufactured by: |
|
Northstar Rx LLC. |
Apotex Inc |
|
Memphis, TN 38141 |
Toronto, Ontario |
|
Canada M9L 1T9 |
December 2021
DESCRIPTION SECTION
DESCRIPTION
The active ingredient in ipratropium bromide nasal solution is ipratropium bromide monohydrate. It is an anticholinergic agent chemically described as 8-azoniabicyclo (3.2.1) octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide, monohydrate (endo, syn)-, (+)-: a synthetic quaternary ammonium compound, chemically related to atropine. The structural formula is:
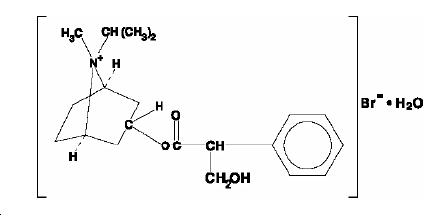
ipratropium bromide C20H30BrNO3•H2O
monohydrate Mol. Wt. 430.4
Ipratropium bromide is a white to off-white, crystalline substance. It is freely soluble in lower alcohols and water, existing in an ionized state in aqueous solutions, and relatively insoluble in non-polar media.
Ipratropium bromide nasal solution, 0.03% is a metered-dose, manual pump spray unit which delivers 21 mcg (70 mcL) ipratropium bromide per spray on an anhydrous basis in an isotonic, aqueous solution. It also contains the following inactive ingredients: benzalkonium chloride, edetate disodium, purified water and sodium chloride. Hydrochloric acid and/or sodium hydroxide may be added to adjust the pH to 4.5 to 4.9. Each bottle contains 345 sprays.
