JANUVIA
These highlights do not include all the information needed to use JANUVIA safely and effectively. See full prescribing information for JANUVIA. JANUVIA (sitagliptin) tablets, for oral useInitial U.S. Approval: 2006
62c4f58b-2b28-4825-aeb6-213ab9b7a026
HUMAN PRESCRIPTION DRUG LABEL
Jun 16, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
sitagliptin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
JANUVIA® is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Limitations of Use
JANUVIA should not be used in patients with type 1 diabetes.
JANUVIA has not been studied in patients with a history of pancreatitis. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using JANUVIA. [See Warnings and Precautions (5.1).]
JANUVIA is a dipeptidyl peptidase-4 (DPP-4) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1)
Limitations of Use:
- JANUVIA should not be used in patients with type 1 diabetes (1)
- JANUVIA has not been studied in patients with a history of pancreatitis. (1, 5.1)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Pancreatitis
There have been postmarketing reports of acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, in patients taking JANUVIA. After initiation of JANUVIA, patients should be observed carefully for signs and symptoms of pancreatitis. If pancreatitis is suspected, JANUVIA should promptly be discontinued and appropriate management should be initiated. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using JANUVIA.
5.2 Heart Failure
An association between dipeptidyl peptidase-4 (DPP-4) inhibitor treatment and heart failure has been observed in cardiovascular outcomes trials for two other members of the DPP-4 inhibitor class. These trials evaluated patients with type 2 diabetes mellitus and atherosclerotic cardiovascular disease.
Consider the risks and benefits of JANUVIA prior to initiating treatment in patients at risk for heart failure, such as those with a prior history of heart failure and a history of renal impairment, and observe these patients for signs and symptoms of heart failure during therapy. Advise patients of the characteristic symptoms of heart failure and to immediately report such symptoms. If heart failure develops, evaluate and manage according to current standards of care and consider discontinuation of JANUVIA.
5.3 Acute Renal Failure
There have been postmarketing reports of worsening renal function, including acute renal failure, sometimes requiring dialysis. A subset of these reports involved patients with renal impairment, some of whom were prescribed inappropriate doses of sitagliptin. A return to baseline levels of renal impairment has been observed with supportive treatment and discontinuation of potentially causative agents. Consideration can be given to cautiously reinitiating JANUVIA if another etiology is deemed likely to have precipitated the acute worsening of renal function.
Assessment of renal function is recommended prior to initiating JANUVIA and periodically thereafter. A dosage adjustment is recommended in patients with moderate or severe renal impairment and in patients with ESRD requiring hemodialysis or peritoneal dialysis. [See Dosage and Administration (2.2); Use in Specific Populations (8.6).]
5.4 Hypoglycemia with Concomitant Use with Insulin or Insulin Secretagogues
When JANUVIA was used in combination with insulin or insulin secretagogues (e.g., sulfonylurea), medications known to cause hypoglycemia, the incidence of hypoglycemia was increased over that of placebo used in combination with a sulfonylurea or with insulin. [See Adverse Reactions (6.1).] Therefore, a lower dose of sulfonylurea or insulin may be required to reduce the risk of hypoglycemia. [See Drug Interactions (7.1).]
5.5 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions in patients treated with JANUVIA. These reactions include anaphylaxis, angioedema, and exfoliative skin conditions including Stevens-Johnson syndrome. Onset of these reactions occurred within the first 3 months after initiation of treatment with JANUVIA, with some reports occurring after the first dose. If a hypersensitivity reaction is suspected, discontinue JANUVIA, assess for other potential causes for the event, and institute alternative treatment for diabetes. [See Adverse Reactions (6.2).]
Angioedema has also been reported with other DPP-4 inhibitors. Use caution in a patient with a history of angioedema with another DPP-4 inhibitor because it is unknown whether such patients will be predisposed to angioedema with JANUVIA.
5.6 Severe and Disabling Arthralgia
There have been postmarketing reports of severe and disabling arthralgia in patients taking DPP-4 inhibitors. The time to onset of symptoms following initiation of drug therapy varied from one day to years. Patients experienced relief of symptoms upon discontinuation of the medication. A subset of patients experienced a recurrence of symptoms when restarting the same drug or a different DPP-4 inhibitor. Consider DPP-4 inhibitors as a possible cause for severe joint pain and discontinue drug if appropriate.
5.7 Bullous Pemphigoid
Postmarketing cases of bullous pemphigoid requiring hospitalization have been reported with DPP-4 inhibitor use. In reported cases, patients typically recovered with topical or systemic immunosuppressive treatment and discontinuation of the DPP-4 inhibitor. Tell patients to report development of blisters or erosions while receiving JANUVIA. If bullous pemphigoid is suspected, JANUVIA should be discontinued and referral to a dermatologist should be considered for diagnosis and appropriate treatment.
- Pancreatitis: There have been postmarketing reports of acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis. If pancreatitis is suspected, promptly discontinue JANUVIA. (5.1)
- Heart failure: Heart failure has been observed with two other members of the DPP-4 inhibitor class. Consider risks and benefits of JANUVIA in patients who have known risk factors for heart failure. Monitor patients for signs and symptoms. (5.2)
- Acute Renal Failure: Has been reported postmarketing, sometimes requiring dialysis. Assessment of renal function is recommended prior to initiating JANUVIA and periodically thereafter. (5.3)
- Hypoglycemia with Concomitant Use with Insulin or Insulin Secretagogues: Increased risk of hypoglycemia when used in combination with insulin and/or an insulin secretagogue. Lower dose of insulin or insulin secretagogue may be required. (5.4, 7.1)
- Hypersensitivity Reactions: There have been postmarketing reports of serious allergic and hypersensitivity reactions in patients treated with JANUVIA such as anaphylaxis, angioedema, and exfoliative skin conditions including Stevens-Johnson syndrome. Promptly stop JANUVIA, assess for other potential causes, institute appropriate monitoring and treatment. (5.5, 6.2)
- Severe and Disabling Arthralgia: Has been reported in patients taking DPP-4 inhibitors. Consider as a possible cause for severe joint pain and discontinue drug if appropriate. (5.6)
- Bullous Pemphigoid: There have been postmarketing reports requiring hospitalization in patients taking DPP-4 inhibitors. Tell patients to report development of blisters or erosions. If bullous pemphigoid is suspected, discontinue JANUVIA. (5.7)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are also discussed elsewhere in the labeling:
- Pancreatitis [see Warnings and Precautions (5.1)]
- Heart Failure [see Warnings and Precautions (5.2)]
- Acute Renal Failure [see Warnings and Precautions (5.3)]
- Hypoglycemia with Concomitant Use with Insulin or Insulin Secretagogues [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
- Severe and Disabling Arthralgia [see Warnings and Precautions (5.6)]
- Bullous Pemphigoid [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled clinical studies as both monotherapy and combination therapy with metformin, pioglitazone, or rosiglitazone and metformin, the overall incidence of adverse reactions, hypoglycemia, and discontinuation of therapy due to clinical adverse reactions with JANUVIA were similar to placebo. In combination with glimepiride, with or without metformin, the overall incidence of clinical adverse reactions with JANUVIA was higher than with placebo, in part related to a higher incidence of hypoglycemia (see Table 3); the incidence of discontinuation due to clinical adverse reactions was similar to placebo.
Two placebo-controlled monotherapy studies, one of 18- and one of 24-week duration, included patients treated with JANUVIA 100 mg daily, JANUVIA 200 mg daily, and placebo. Five placebo-controlled add-on combination therapy studies were also conducted: one with metformin; one with pioglitazone; one with metformin and rosiglitazone; one with glimepiride (with or without metformin); and one with insulin (with or without metformin). In these trials, patients with inadequate glycemic control on a stable dose of the background therapy were randomized to add-on therapy with JANUVIA 100 mg daily or placebo. The adverse reactions, excluding hypoglycemia, reported regardless of investigator assessment of causality in ≥5% of patients treated with JANUVIA 100 mg daily and more commonly than in patients treated with placebo, are shown in Table 1 for the clinical trials of at least 18 weeks duration. Incidences of hypoglycemia are shown in Table 3.
Table 1: Placebo-Controlled Clinical Studies of JANUVIA Monotherapy or Add-on Combination Therapy with Pioglitazone, Metformin + Rosiglitazone, or Glimepiride +/- Metformin: Adverse Reactions (Excluding Hypoglycemia) Reported in ≥5% of Patients and More Commonly than in Patients Given Placebo, Regardless of Investigator Assessment of Causality*|
Number of Patients (%) | ||
| ||
|
Monotherapy (18 or 24 weeks) |
JANUVIA 100 mg |
Placebo |
|
N = 443 |
N = 363 | |
|
Nasopharyngitis |
23 (5.2) |
12 (3.3) |
|
Combination with Pioglitazone (24 |
JANUVIA 100 mg + Pioglitazone |
Placebo + Pioglitazone |
|
N = 175 |
N = 178 | |
|
Upper Respiratory Tract Infection |
11 (6.3) |
6 (3.4) |
|
Headache |
9 (5.1) |
7 (3.9) |
|
Combination with Metformin + |
JANUVIA 100 mg + Metformin |
Placebo + Metformin**** |
|
N = 181 |
N = 97 | |
|
Upper Respiratory Tract Infection |
10 (5.5) |
5 (5.2) |
|
Nasopharyngitis |
11 (6.1) |
4 (4.1) |
|
Combination with Glimepiride |
JANUVIA 100 mg + Glimepiride (+/- Metformin) |
Placebo + Glimepiride (+/- Metformin) |
|
N = 222 |
N = 219 | |
|
Nasopharyngitis |
14 (6.3) |
10 (4.6) |
|
Headache |
13 (5.9) |
5 (2.3) |
In the 24-week study of patients receiving JANUVIA as add-on combination therapy with metformin, there were no adverse reactions reported regardless of investigator assessment of causality in ≥5% of patients and more commonly than in patients given placebo.
In the 24-week study of patients receiving JANUVIA as add-on therapy to insulin (with or without metformin), there were no adverse reactions reported regardless of investigator assessment of causality in ≥5% of patients and more commonly than in patients given placebo, except for hypoglycemia (see Table 3).
In the study of JANUVIA as add-on combination therapy with metformin and rosiglitazone (Table 1), through Week 54 the adverse reactions reported regardless of investigator assessment of causality in ≥5% of patients treated with JANUVIA and more commonly than in patients treated with placebo were: upper respiratory tract infection (JANUVIA, 15.5%; placebo, 6.2%), nasopharyngitis (11.0%, 9.3%), peripheral edema (8.3%, 5.2%), and headache (5.5%, 4.1%).
In a pooled analysis of the two monotherapy studies, the add-on to metformin study, and the add-on to pioglitazone study, the incidence of selected gastrointestinal adverse reactions in patients treated with JANUVIA was as follows: abdominal pain (JANUVIA 100 mg, 2.3%; placebo, 2.1%), nausea (1.4%, 0.6%), and diarrhea (3.0%, 2.3%).
In an additional, 24-week, placebo-controlled factorial study of initial therapy with sitagliptin in combination with metformin, the adverse reactions reported (regardless of investigator assessment of causality) in ≥5% of patients are shown in Table 2.
Table 2: Initial Therapy with Combination of Sitagliptin and Metformin: Adverse Reactions Reported (Regardless of Investigator Assessment of Causality) in ≥5% of Patients Receiving Combination Therapy (and Greater than in Patients Receiving Metformin alone, Sitagliptin alone, and Placebo)*|
Number of Patients (%) | ||||
| ||||
|
Placebo |
Sitagliptin (JANUVIA) |
Metformin HCl 500 or 1000 mg bid**†** |
Sitagliptin 50 mg bid + Metformin HCl 500 or 1000 mg bid**†** | |
|
N = 176 |
N = 179 |
N = 364† |
N = 372† | |
|
Upper Respiratory Infection |
9 (5.1) |
8 (4.5) |
19 (5.2) |
23 (6.2) |
|
Headache |
5 (2.8) |
2 (1.1) |
14 (3.8) |
22 (5.9) |
In a 24-week study of initial therapy with JANUVIA in combination with pioglitazone, there were no adverse reactions reported (regardless of investigator assessment of causality) in ≥5% of patients and more commonly than in patients given pioglitazone alone.
No clinically meaningful changes in vital signs or in ECG (including in QTc interval) were observed in patients treated with JANUVIA.
In a pooled analysis of 19 double-blind clinical trials that included data from 10,246 patients randomized to receive sitagliptin 100 mg/day (N=5429) or corresponding (active or placebo) control (N=4817), the incidence of acute pancreatitis was 0.1 per 100 patient-years in each group (4 patients with an event in 4708 patient-years for sitagliptin and 4 patients with an event in 3942 patient-years for control).
Hypoglycemia
In the above studies (N=9), adverse reactions of hypoglycemia were based on all reports of symptomatic hypoglycemia. A concurrent blood glucose measurement was not required although most (74%) reports of hypoglycemia were accompanied by a blood glucose measurement ≤70 mg/dL. When JANUVIA was coadministered with a sulfonylurea or with insulin, the percentage of patients with at least one adverse reaction of hypoglycemia was higher than in the corresponding placebo group (Table 3).
Table 3: Incidence and Rate of Hypoglycemia* in Placebo-Controlled Clinical Studies when JANUVIA was used as Add-On Therapy to Glimepiride (with or without Metformin) or Insulin (with or without Metformin), Regardless of Investigator Assessment of Causality|
Add-On to Glimepiride |
JANUVIA 100 mg + Glimepiride (+/- Metformin) |
Placebo + Glimepiride (+/- Metformin) |
| ||
|
N = 222 |
N = 219 | |
|
Overall (%) |
27 (12.2) |
4 (1.8) |
|
Rate (episodes/patient-year)† |
0.59 |
0.24 |
|
Severe (%)‡ |
0 (0.0) |
0 (0.0) |
|
Add-On to Insulin |
JANUVIA 100 mg + Insulin (+/- Metformin) |
Placebo + Insulin (+/- Metformin) |
|
N = 322 |
N = 319 | |
|
Overall (%) |
50 (15.5) |
25 (7.8) |
|
Rate (episodes/patient-year)† |
1.06 |
0.51 |
|
Severe (%)‡ |
2 (0.6) |
1 (0.3) |
In a pooled analysis of the two monotherapy studies, the add-on to metformin study, and the add-on to pioglitazone study, the overall incidence of adverse reactions of hypoglycemia was 1.2% in patients treated with JANUVIA 100 mg and 0.9% in patients treated with placebo.
In the study of JANUVIA as add-on combination therapy with metformin and rosiglitazone, the overall incidence of hypoglycemia was 2.2% in patients given add-on JANUVIA and 0.0% in patients given add-on placebo through Week 18. Through Week 54, the overall incidence of hypoglycemia was 3.9% in patients given add-on JANUVIA and 1.0% in patients given add-on placebo.
In the 24-week, placebo-controlled factorial study of initial therapy with JANUVIA in combination with metformin, the incidence of hypoglycemia was 0.6% in patients given placebo, 0.6% in patients given JANUVIA alone, 0.8% in patients given metformin alone, and 1.6% in patients given JANUVIA in combination with metformin.
In the study of JANUVIA as initial therapy with pioglitazone, one patient taking JANUVIA experienced a severe episode of hypoglycemia. There were no severe hypoglycemia episodes reported in other studies except in the study involving coadministration with insulin.
In an additional, 30-week placebo-controlled, study of patients with type 2 diabetes inadequately controlled with metformin comparing the maintenance of sitagliptin 100 mg versus withdrawal of sitagliptin when initiating basal insulin therapy, the event rate and incidence of documented symptomatic hypoglycemia (blood glucose measurement ≤70 mg/dL) did not differ between the sitagliptin and placebo groups.
Laboratory Tests
Across clinical studies, the incidence of laboratory adverse reactions was similar in patients treated with JANUVIA 100 mg compared to patients treated with placebo. A small increase in white blood cell count (WBC) was observed due to an increase in neutrophils. This increase in WBC (of approximately 200 cells/microL vs placebo, in four pooled placebo-controlled clinical studies, with a mean baseline WBC count of approximately 6600 cells/microL) is not considered to be clinically relevant. In a 12-week study of 91 patients with chronic renal insufficiency, 37 patients with moderate renal insufficiency were randomized to JANUVIA 50 mg daily, while 14 patients with the same magnitude of renal impairment were randomized to placebo. Mean (SE) increases in serum creatinine were observed in patients treated with JANUVIA [0.12 mg/dL (0.04)] and in patients treated with placebo [0.07 mg/dL (0.07)]. The clinical significance of this added increase in serum creatinine relative to placebo is not known.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during postapproval use of JANUVIA as monotherapy and/or in combination with other antihyperglycemic agents. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity reactions including anaphylaxis, angioedema, rash, urticaria, cutaneous vasculitis, and exfoliative skin conditions including Stevens- Johnson syndrome; hepatic enzyme elevations; acute pancreatitis, including fatal and non-fatal hemorrhagic and necrotizing pancreatitis [see Indications and Usage (1)]; worsening renal function, including acute renal failure (sometimes requiring dialysis), and tubulointerstitial nephritis; severe and disabling arthralgia; bullous pemphigoid; constipation; vomiting; headache; myalgia; pain in extremity; back pain; pruritus; mouth ulceration; stomatitis; rhabdomyolysis.
Adverse reactions reported in ≥5% of patients treated with JANUVIA and more commonly than in patients treated with placebo are: upper respiratory tract infection, nasopharyngitis and headache. In the add-on to sulfonylurea and add-on to insulin studies, hypoglycemia was also more commonly reported in patients treated with JANUVIA compared to placebo. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Insulin Secretagogues or Insulin
Coadministration of JANUVIA with an insulin secretagogue (e.g., sulfonylurea) or insulin may require lower doses of the insulin secretagogue or insulin to reduce the risk of hypoglycemia. [See Warnings and Precautions (5.4).]
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
The recommended dose of JANUVIA is 100 mg once daily. JANUVIA can be taken with or without food.
2.2 Recommendations for Use in Renal Impairment
Assess renal function prior to initiation of JANUVIA and periodically thereafter.
For patients with an estimated glomerular filtration rate [eGFR] greater than or equal to 45 mL/min/1.73 m2 to less than 90 mL/min/1.73 m2, no dosage adjustment for JANUVIA is required.
For patients with moderate renal impairment (eGFR greater than or equal to 30 mL/min/1.73 m2 to less than 45 mL/min/1.73 m2), the dose of JANUVIA is 50 mg once daily.
For patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2) or with end-stage renal disease (ESRD) requiring hemodialysis or peritoneal dialysis, the dose of JANUVIA is 25 mg once daily. JANUVIA may be administered without regard to the timing of dialysis.
The recommended dose of JANUVIA is 100 mg once daily. JANUVIA can be taken with or without food. (2.1)
Dosage adjustment is recommended for patients with eGFR less than 45 mL/min/1.73 m2. (2.2)
Dosage Adjustment in Patients with Renal Impairment (2.2)|
eGFR greater than or equal to 30 mL/min/1.73 m2 to less than 45 mL/min/1.73 m2 |
eGFR less than 30 mL/min/1.73 m2 (including patients with end stage renal disease [ESRD] on dialysis) |
|
50 mg once daily |
25 mg once daily |
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sitagliptin is a DPP-4 inhibitor, which is believed to exert its actions in patients with type 2 diabetes mellitus by slowing the inactivation of incretin hormones. Concentrations of the active intact hormones are increased by sitagliptin, thereby increasing and prolonging the action of these hormones. Incretin hormones, including glucagon-like peptide-1 (GLP-1) and glucose- dependent insulinotropic polypeptide (GIP), are released by the intestine throughout the day, and levels are increased in response to a meal. These hormones are rapidly inactivated by the enzyme, DPP-4. The incretins are part of an endogenous system involved in the physiologic regulation of glucose homeostasis. When blood glucose concentrations are normal or elevated, GLP-1 and GIP increase insulin synthesis and release from pancreatic beta cells by intracellular signaling pathways involving cyclic AMP. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, leading to reduced hepatic glucose production. By increasing and prolonging active incretin levels, sitagliptin increases insulin release and decreases glucagon levels in the circulation in a glucose-dependent manner. Sitagliptin demonstrates selectivity for DPP-4 and does not inhibit DPP-8 or DPP-9 activity in vitro at concentrations approximating those from therapeutic doses.
12.2 Pharmacodynamics
General
In patients with type 2 diabetes mellitus, administration of sitagliptin led to inhibition of DPP-4 enzyme activity for a 24-hour period. After an oral glucose load or a meal, this DPP-4 inhibition resulted in a 2- to 3-fold increase in circulating levels of active GLP-1 and GIP, decreased glucagon concentrations, and increased responsiveness of insulin release to glucose, resulting in higher C-peptide and insulin concentrations. The rise in insulin with the decrease in glucagon was associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal.
In studies with healthy subjects, sitagliptin did not lower blood glucose or cause hypoglycemia.
Sitagliptin and Metformin hydrochloride Coadministration
In a two-day study in healthy subjects, sitagliptin alone increased active GLP-1 concentrations, whereas metformin alone increased active and total GLP-1 concentrations to similar extents. Coadministration of sitagliptin and metformin had an additive effect on active GLP-1 concentrations. Sitagliptin, but not metformin, increased active GIP concentrations. It is unclear how these findings relate to changes in glycemic control in patients with type 2 diabetes mellitus.
Cardiac Electrophysiology
In a randomized, placebo-controlled crossover study, 79 healthy subjects were administered a single oral dose of sitagliptin 100 mg, sitagliptin 800 mg (8 times the recommended dose), and placebo. At the recommended dose of 100 mg, there was no effect on the QTc interval obtained at the peak plasma concentration, or at any other time during the study. Following the 800 mg dose, the maximum increase in the placebo-corrected mean change in QTc from baseline was observed at 3 hours postdose and was 8.0 msec. This increase is not considered to be clinically significant. At the 800 mg dose, peak sitagliptin plasma concentrations were approximately 11 times higher than the peak concentrations following a 100-mg dose.
In patients with type 2 diabetes mellitus administered sitagliptin 100 mg (N=81) or sitagliptin 200 mg (N=63) daily, there were no meaningful changes in QTc interval based on ECG data obtained at the time of expected peak plasma concentration.
12.3 Pharmacokinetics
The pharmacokinetics of sitagliptin have been extensively characterized in healthy subjects and patients with type 2 diabetes mellitus. Following a single oral 100-mg dose to healthy volunteers, mean plasma AUC of sitagliptin was 8.52 μM•hr, Cmax was 950 nM, and apparent terminal half-life (t1/2) was 12.4 hours. Plasma AUC of sitagliptin increased in a dose-proportional manner and increased approximately 14% following 100 mg doses at steady-state compared to the first dose. The intra-subject and inter-subject coefficients of variation for sitagliptin AUC were small (5.8% and 15.1%). The pharmacokinetics of sitagliptin was generally similar in healthy subjects and in patients with type 2 diabetes mellitus.
Absorption
After oral administration of a 100 mg dose to healthy subjects, sitagliptin was rapidly absorbed with peak plasma concentrations (median Tmax) occurring 1 to 4 hours postdose. The absolute bioavailability of sitagliptin is approximately 87%.
Effect of Food
Coadministration of a high-fat meal with sitagliptin had no effect on the pharmacokinetics of sitagliptin.
Distribution
The mean volume of distribution at steady state following a single 100-mg intravenous dose of sitagliptin to healthy subjects is approximately 198 liters. The fraction of sitagliptin reversibly bound to plasma proteins is low (38%).
Elimination
Approximately 79% of sitagliptin is excreted unchanged in the urine with metabolism being a minor pathway of elimination. The apparent terminal t1/2 following a 100 mg oral dose of sitagliptin was approximately 12.4 hours and renal clearance was approximately 350 mL/min.
Metabolism
Following a [14C]sitagliptin oral dose, approximately 16% of the radioactivity was excreted as metabolites of sitagliptin. Six metabolites were detected at trace levels and are not expected to contribute to the plasma DPP-4 inhibitory activity of sitagliptin. In vitro studies indicated that the primary enzyme responsible for the limited metabolism of sitagliptin was CYP3A4, with contribution from CYP2C8.
Excretion
Following administration of an oral [14C]sitagliptin dose to healthy subjects, approximately 100% of the administered radioactivity was eliminated in feces (13%) or urine (87%) within one week of dosing.
Elimination of sitagliptin occurs primarily via renal excretion and involves active tubular secretion. Sitagliptin is a substrate for human organic anion transporter-3 (hOAT-3), which may be involved in the renal elimination of sitagliptin. The clinical relevance of hOAT-3 in sitagliptin transport has not been established. Sitagliptin is also a substrate of P-glycoprotein (P-gp), which may also be involved in mediating the renal elimination of sitagliptin. However, cyclosporine, a P-gp inhibitor, did not reduce the renal clearance of sitagliptin.
Specific Populations
Patients with Renal Impairment
An approximately 2-fold increase in the plasma AUC of sitagliptin was observed in patients with moderate renal impairment with eGFR of 30 to less than 45 mL/min/1.73 m2, and an approximately 4-fold increase was observed in patients with severe renal impairment, including patients with ESRD on hemodialysis, as compared to normal healthy control subjects.
Patients with Hepatic Impairment
In patients with moderate hepatic impairment (Child-Pugh score 7 to 9), mean AUC and Cmax of sitagliptin increased approximately 21% and 13%, respectively, compared to healthy matched controls following administration of a single 100-mg dose of sitagliptin. These differences are not considered to be clinically meaningful.
There is no clinical experience in patients with severe hepatic impairment (Child-Pugh score >9).
Effects of Age, Body Mass Index (BMI), Gender, and Race
Based on a population pharmacokinetic analysis or a composite analysis of available pharmacokinetic data, BMI, gender, and race do not have a clinically meaningful effect on the pharmacokinetics of sitagliptin. When the effects of age on renal function are taken into account, age alone did not have a clinically meaningful impact on the pharmacokinetics of sitagliptin based on a population pharmacokinetic analysis. Elderly subjects (65 to 80 years) had approximately 19% higher plasma concentrations of sitagliptin compared to younger subjects.
Drug Interaction Studies
In Vitro Assessment of Drug Interactions
Sitagliptin is not an inhibitor of CYP isozymes CYP3A4, 2C8, 2C9, 2D6, 1A2, 2C19 or 2B6, and is not an inducer of CYP3A4. Sitagliptin is a P-gp substrate, but does not inhibit P-gp mediated transport of digoxin. Based on these results, sitagliptin is considered unlikely to cause interactions with other drugs that utilize these pathways.
Sitagliptin is not extensively bound to plasma proteins. Therefore, the propensity of sitagliptin to be involved in clinically meaningful drug-drug interactions mediated by plasma protein binding displacement is very low.
In Vivo Assessment of Drug Interactions
Effects of Sitagliptin on Other Drugs
In clinical studies, sitagliptin did not meaningfully alter the pharmacokinetics of metformin, glyburide, simvastatin, rosiglitazone, digoxin, warfarin, or an oral contraceptive (ethinyl estradiol and norethindrone) (Table 4), providing in vivo evidence of a low propensity for causing drug interactions with substrates of CYP3A4, CYP2C8, CYP2C9, P-gp, and organic cationic transporter (OCT).
Table 4: Effect of Sitagliptin on Systemic Exposure of Coadministered Drugs|
Coadministered Drug |
Dose of Coadministered Drug* |
Dose of Sitagliptin* |
Geometric Mean Ratio | ||
|---|---|---|---|---|---|
|
AUC† |
Cmax | ||||
| |||||
|
Digoxin |
0.25 mg‡ once daily for 10 days |
100 mg‡ once daily for 10 days |
Digoxin |
1.11§ |
1.18 |
|
Glyburide |
1.25 mg |
200 mg‡ once daily for 6 days |
Glyburide |
1.09 |
1.01 |
|
Simvastatin |
20 mg |
200 mg‡ once daily for 5 days |
Simvastatin |
0.85¶ |
0.80 |
|
Simvastatin Acid |
1.12¶ |
1.06 | |||
|
Rosiglitazone |
4 mg |
200 mg‡ once daily for 5 days |
Rosiglitazone |
0.98 |
0.99 |
|
Warfarin |
30 mg single dose on day 5 |
200 mg‡ once daily for 11 days |
S(-) Warfarin |
0.95 |
0.89 |
|
R(+) Warfarin |
0.99 |
0.89 | |||
|
Ethinyl estradiol and norethindrone |
21 days once daily of 35 µg ethinyl estradiol with norethindrone 0.5 mg × 7 days, 0.75 mg × 7 days, 1.0 mg × 7 days |
200 mg‡ once daily for 21 days |
Ethinyl estradiol |
0.99 |
0.97 |
|
Norethindrone |
1.03 |
0.98 | |||
|
Metformin HCl |
1000 mg‡ twice daily for 14 days |
50 mg‡ twice daily for 7 days |
Metformin |
1.02# |
0.97 |
Effects of Other Drugs on Sitagliptin
Clinical data described below suggest that sitagliptin is not susceptible to clinically meaningful interactions by coadministered medications (Table 5).
Table 5: Effect of Coadministered Drugs on Systemic Exposure of Sitagliptin|
Coadministered Drug |
Dose of Coadministered Drug* |
Dose of Sitagliptin* |
Geometric Mean Ratio | ||
|---|---|---|---|---|---|
|
AUC† |
Cmax | ||||
| |||||
|
Cyclosporine |
600 mg once daily |
100 mg once daily |
Sitagliptin |
1.29 |
1.68 |
|
Metformin HCl |
1000 mg‡ twice daily for 14 days |
50 mg‡ twice daily for 7 days |
Sitagliptin |
1.02§ |
1.05 |
SPL MEDGUIDE SECTION
|
Medication Guide | ||||||
|---|---|---|---|---|---|---|
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Revised: 07/2022 | |||||
|
Read this Medication Guide carefully before you start taking JANUVIA and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about JANUVIA, ask your doctor or pharmacist. | ||||||
|
What is the most important information I should know about JANUVIA? *Inflammation of the pancreas (pancreatitis) which may be severe and lead to death. Certain medical problems make you more likely to get pancreatitis. | ||||||
|
|
| ||||
|
Stop taking JANUVIA and call your doctor right away if you have pain in your stomach area (abdomen) that is severe and will not go away. The pain may be felt going from your abdomen through to your back. The pain may happen with or without vomiting. These may be symptoms of pancreatitis. | ||||||
|
*Heart failure. Heart failure means your heart does not pump blood well enough. | ||||||
These may be symptoms of heart failure. | ||||||
|
What is JANUVIA?
| ||||||
|
Who should not take JANUVIA?
Symptoms of a serious allergic reaction to JANUVIA may include rash, raised red patches on your skin (hives), or swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing. | ||||||
|
What should I tell my doctor before taking JANUVIA?
Tell your doctor about all the medicines you take, including prescription
and over-the-counter medicines, vitamins, and herbal supplements. | ||||||
|
How should I take JANUVIA?
| ||||||
|
What are the possible side effects of JANUVIA?
| ||||||
|
|
|
|
| ||
|
*Serious allergic reactions. If you have any symptoms of a serious allergic reaction, stop taking JANUVIA and call your doctor right away or get emergency medical help. See "Who should not take JANUVIA?". Your doctor may give you a medicine for your allergic reaction and prescribe a different medicine for your diabetes. *Joint pain. Some people who take medicines called DPP-4 inhibitors like JANUVIA, may develop joint pain that can be severe. Call your doctor if you have severe joint pain. *Skin reaction. Some people who take medicines called DPP-4 inhibitors like JANUVIA may develop a skin reaction called bullous pemphigoid that can require treatment in a hospital. Tell your doctor right away if you develop blisters or the breakdown of the outer layer of your skin (erosion). Your doctor may tell you to stop taking JANUVIA. The most common side effects of JANUVIA include upper respiratory infection,
stuffy or runny nose and sore throat, and headache. | ||||||
|
How should I store JANUVIA? | ||||||
|
General information about the safe and effective use of JANUVIA. | ||||||
|
What are the ingredients in JANUVIA? | ||||||
|
Distributed by: Merck Sharp & Dohme LLC |
DESCRIPTION SECTION
11 DESCRIPTION
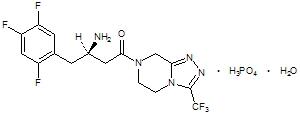
JANUVIA Tablets contain sitagliptin phosphate, an orally-active inhibitor of the dipeptidyl peptidase-4 (DPP-4) enzyme.
Sitagliptin phosphate monohydrate is described chemically as 7-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazine phosphate (1:1) monohydrate.
The empirical formula is C16H15F6N5O•H3PO4•H2O and the molecular weight is 523.32. The structural formula is:
|
|
Sitagliptin phosphate monohydrate is a white to off-white, crystalline, non- hygroscopic powder. It is soluble in water and N,N-dimethyl formamide; slightly soluble in methanol; very slightly soluble in ethanol, acetone, and acetonitrile; and insoluble in isopropanol and isopropyl acetate.
Each film-coated tablet of JANUVIA contains 32.13, 64.25, or 128.5 mg of sitagliptin phosphate monohydrate, which is equivalent to 25, 50, or 100 mg, respectively, of free base and the following inactive ingredients: microcrystalline cellulose, anhydrous dibasic calcium phosphate, croscarmellose sodium, magnesium stearate, and sodium stearyl fumarate. In addition, the film coating contains the following inactive ingredients: polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, red iron oxide, and yellow iron oxide.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Pancreatitis
Inform patients that acute pancreatitis has been reported during postmarketing use of JANUVIA. Inform patients that persistent severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to promptly discontinue JANUVIA and contact their physician if persistent severe abdominal pain occurs [see Warnings and Precautions (5.1)].
Heart Failure
Inform patients of the signs and symptoms of heart failure. Before initiating JANUVIA, ask patients about a history of heart failure or other risk factors for heart failure including moderate to severe renal impairment. Instruct patients to contact their health care provider as soon as possible if they experience symptoms of heart failure, including increasing shortness of breath, rapid increase in weight or swelling of the feet [see Warnings and Precautions (5.2)].
Hypoglycemia
Inform patients that the incidence of hypoglycemia is increased when JANUVIA is added to a sulfonylurea or insulin. Explain to patients receiving JANUVIA in combination with these medications the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development [see Warnings and Precautions (5.4)].
Hypersensitivity Reactions
Inform patients that allergic reactions have been reported during postmarketing use of JANUVIA. If symptoms of allergic reactions (including rash, hives, and swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing) occur, patients must stop taking JANUVIA and seek medical advice promptly [see Warnings and Precautions (5.5)].
Severe and Disabling Arthralgia
Inform patients that severe and disabling joint pain may occur with this class of drugs. The time to onset of symptoms can range from one day to years. Instruct patients to seek medical advice if severe joint pain occurs [see Warnings and Precautions (5.6)].
Bullous Pemphigoid
Inform patients that bullous pemphigoid may occur with this class of drugs. Instruct patients to seek medical advice if blisters or erosions occur [see Warnings and Precautions (5.7)].