Cefdinir
CEFDINIR CAPSULES USP
86c21cdd-8b94-f4dc-e053-2991aa0a51eb
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
NuCare Pharmaceuticals,Inc.
DUNS: 010632300
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Cefdinir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL

DESCRIPTION SECTION
DESCRIPTION
Cefdinir capsules contains the active ingredient cefdinir, an extended- spectrum, semisynthetic cephalosporin, for oral administration. Chemically, cefdinir is [6R-[6α,7β(Z)]]-7-[[(2-amino-4 thiazolyl) (hydroxyimino) acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Cefdinir is a white to slightly brownish-yellow solid. It is slightly soluble in dilute hydrochloric acid and sparingly soluble in 0.1 M pH 7.0 phosphate buffer. The molecular formula is C 14H 13N 5O 5S 2 and the molecular weight is 395.42. Cefdinir has the structural formula shown below:
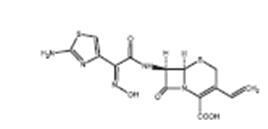
Cefdinir capsules contain 300 mg of cefdinir and the following inactive ingredients: carboxymethylcellulose calcium; colloidal silicon dioxide; and magnesium stearate. The capsule shells contain D&C Red #28; FD&C Blue #1; FD&C Red #40; gelatin and titanium dioxide.
HOW SUPPLIED SECTION
HOW SUPPLIED
Cefdinir capsules USP, 300 mg, size 0 capsules having blue cap imprinted twice with LUPIN (in black ink) and purple body imprinted twice with CEFDINIR (in white ink) containing off white to creamish granular slug, are available as follows:
NDC 68071-4856-7 BOTTLES OF 14
NDC 68071-4856-2 BOTTLES OF 20
Store the capsules at 20°-25°C (68°-77°F); [see USP Controlled Room Temperature].
