Psoriasis Scalp Treatment
14ab77e7-86b0-49d3-8efa-8a80c5a24d74
HUMAN OTC DRUG LABEL
May 28, 2025
Rida LLC
DUNS: 004425803
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Salicylic acid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Product label
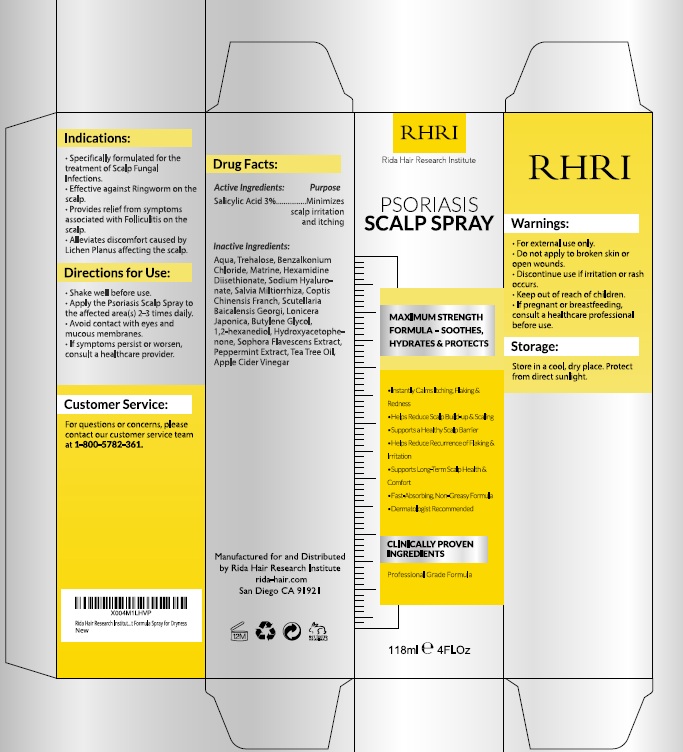
INDICATIONS & USAGE SECTION
Uses
- Specifically formulated for the treatment of scalp fungal infections.
- Effective against ringworm on the scalp.
- Provides relief from symptoms associated with folliculitis on the scalp.
- Alleviates discomfort caused by lichen planus affecting the scalp.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Salicylic Acid 3%
OTC - PURPOSE SECTION
Purpose
Minimizes scalp irritation and itching
WARNINGS SECTION
Warnings
*For external use only.
- Do not apply to broken skin or open wounds.
- Discontinue use if irritation or rash occurs. *Keep out of reach of children.
- If pregnant or breastfeeding, consult a healthcare professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
DOSAGE & ADMINISTRATION SECTION
Directions
- Shake well before use.
- Apply the Folliculitis Scalp Spray to the affected area 2–3 times daily.
- Avoid contact with eyes and mucous membranes.
- If symptoms persist or worsen, consult a healthcare provider.
SPL UNCLASSIFIED SECTION
Other Information
- Store in a cool, dry place. Protect from direct sunlight.
INACTIVE INGREDIENT SECTION
Inactive ingredients
Aqua (Water), Trehalose, Benzalkonium Chloride, Mirtazapine, Hexamidine, Sodium Hyaluronate, Salvia Miltiorrhiza Extract, Ginkgo Biloba Extract, Bacillus Subtilis Ferment, Coptis Chinensis Extract, 1,2-Hexanediol, Hydroxypropyl Guar, Saponins, Apple Cider Vinegar
