DILTIAZEM HYDROCHLORIDE
Diltiazem Hydrochloride Extended-Release Capsules, USP (Once-a-day dosage), 120 mg, 180 mg and 240 mg
56616fc9-f3f7-8e0a-22c8-a6af106e6b1d
HUMAN PRESCRIPTION DRUG LABEL
Sep 17, 2025
NORTHSTAR RX LLC
DUNS: 830546433
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DILTIAZEM HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
DILTIAZEM HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
DILTIAZEM HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL-240 mg
Representative sample of labeling (seeHOW SUPPLIEDsection for complete listing):
Northstar Rx LLC NDC 16714-525-01
Diltiazem Hydrochloride Extended-Release Capsules, USP (Once-a-day dosage)
240 mg
Rx
100 count

DESCRIPTION SECTION
DESCRIPTION
Diltiazem hydrochloride is a calcium ion influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl) monohydrochloride, (+)-cis-. Its molecular formula is C22H26N2O4S HCl and its molecular weight is 450.99. Its structural formula is as follows:
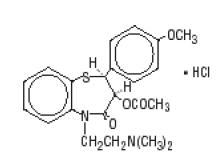
Diltiazem hydrochloride is a white to off-white crystalline powder with a bitter taste. It is soluble in water, methanol and chloroform. Diltiazem Hydrochloride Extended-Release Capsules, USP (Once-a-day dosage) complies with USP Drug Release Test #2.
Diltiazem Hydrochloride Extended-Release Capsules, USP (Once-a-day dosage) contain multiple units of diltiazem HCI extended-release 60 mg, resulting in 120 mg, 180 mg, or 240 mg dosage strengths allowing for the controlled release of diltiazem HCI over a 24-hour period.
Inactive Ingredients
Diltiazem Hydrochloride Extended-Release Capsules, USP (Once-a-day dosage) also contain Black SW-9008/SW9009, colloidal silicon dioxide, D&C Red # No.28, D&C Yellow # No.10, FD&C Blue # No.1, FD&C Red # No.40, gelatin, hypromellose, magnesium stearate, red iron oxide, titanium dioxide and yellow iron oxide.
For oral administration.
