Phexxi
These highlights do not include all the information needed to use PHEXXI safely and effectively. See full prescribing information for PHEXXI. PHEXXI (lactic acid, citric acid, and potassium bitartrate) vaginal gel Initial U.S. Approval: 2020
173ff411-7227-47b0-94dc-844e1ebaf14e
HUMAN PRESCRIPTION DRUG LABEL
Nov 21, 2023
Evofem, Inc.
DUNS: 832466119
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
lactic acid, L-, citric acid monohydrate, and potassium bitartrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 12 Applicator Box
phexxi™
(lactic acid, citric acid, and
potassium bitartrate) Vaginal Gel
1.8%, 1%, 0.4%
NDC 69751-100-12
PN-5011

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Cystitis and Pyelonephritis
Among 2804 subjects who received PHEXXI in Studies 1 and 2, 0.36% (n=10) reported adverse reactions of cystitis, pyelonephritis, or other upper urinary tract infection (UTI). Of these, one case of pyelonephritis was considered serious and required hospitalization. Avoid use of PHEXXI in females of reproductive potential with a history of recurrent urinary tract infection or urinary tract abnormalities.
- Cystitis and Pyelonephritis: Avoid use in women with a history of recurrent UTI or urinary tract abnormalities (5.1)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Administer one pre-filled applicator of PHEXXI (5 grams) vaginally immediately before or up to one hourbefore each act of vaginal intercourse. If more than one act of vaginal intercourse occurs within one hour, an additional dose must be applied. Five grams of PHEXXI contains 90 mg of lactic acid, 50 mg of citric acid, and 20 mg of potassium bitartrate.
2.2 Timing of PHEXXI Use
May use PHEXXI during any part of the menstrual cycle. May use PHEXXI as soon as it is safe to resume vaginal intercourse after childbirth, abortion, or miscarriage.
2.3 Use of PHEXXI with Other Contraceptive Methods
PHEXXI may be used concomitantly with hormonal contraceptives; latex, polyurethane, and polyisoprene condoms; and vaginal diaphragms. Avoid PHEXXI use with vaginal rings.
2.4 Use of PHEXXI with Other Vaginal Products
PHEXXI may be used concomitantly with other products for vaginal infections including miconazole, metronidazole, and tioconazole.
- Administer one (1) pre-filled single-dose applicator of PHEXXI (5 grams) vaginally immediately before (or up to one hour before) each episode of vaginal intercourse (2.1)
- May use during any part of the menstrual cycle (2.2)
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
PHEXXI (lactic acid, citric acid, and potassium bitartrate) vaginal gel is an off-white to tan color gel of uniform consistency containing lactic acid (1.8%), citric acid (1%), and potassium bitartrate (0.4%), supplied as individually wrapped 5 gram pre-filled single-dose vaginal applicators in sealed foil pouches along with a plunger, and are available as follows:
|
Box of 12 units |
|
Sample box of 3 units |
Store in the original foil pack at room temperature 20°C to 25°C (68°F to 77°F); excursion permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
INSTRUCTIONS FOR USE SECTION
INSTRUCTIONS FOR USE
PHEXXI**®**** (FEX ee)**
** (lactic acid, citric acid, and potassium bitartrate) vaginal gel**
For Vaginal Use Only
These Instructions for Use contain information on how to use PHEXXI vaginal gel. Make sure that you read, understand, and follow the Instructions for Use before using PHEXXI and each time you get a refill. There may be new information.
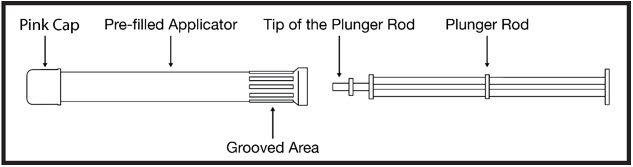
Contents:
- Each box contains either 3 or 12 foil pouches.
- Each foil pouch contains a pre-filled applicator and plunger rod (see Figure A).
- Each pre-filled applicator contains 1 dose of PHEXXI for 1-time use (single use).
|
|
|
Figure A |
Important Information You Need to Know Before Using PHEXXI
- Use 1 dose of PHEXXIwithin 1 hour before you have vaginal sex.
- A new PHEXXI pre-filled applicator must be usedeach time you have vaginal sex. If you have vaginal sex more than 1 time within 1 hour,a new PHEXXI pre-filled applicator must be used.
Prepare to Use PHEXXI
Keep the pre-filled applicator and plunger rod in the foil pouch until you are ready to use PHEXXI.
Step 1: Wash Your Hands
- Wash your hands with soap and water before opening the foil pouch.
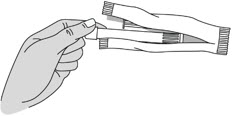
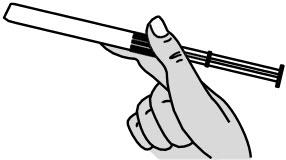
Step 2: Remove the Pre-filled Applicator and Plunger Rod from the Foil Pouch
|
|
|
Figure B |
Important: Do not remove the pink cap until instructed in Step 4.
Insert PHEXXI Gel
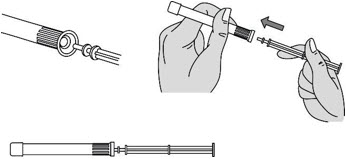
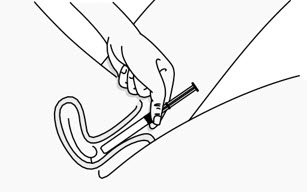
Step 3: Insert the Plunger Rod
|
|
|
Figure C |
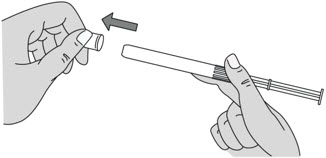
Step 4: Remove the Pink Cap
|
|
|
Figure D |
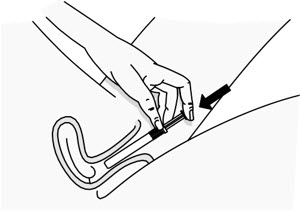
Step 5: Insert the PHEXXI Pre-filled Applicator into the Vagina
|
|
|
Figure E | |
|
| |
|
Figure F |
Step 6: Insert PHEXXI Gel
|
|
|
Figure G |
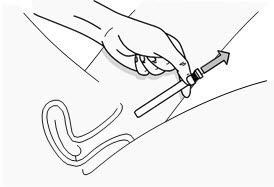
Step 7: Remove the Used PHEXXI Pre-filled Applicator
|
|
|
Figure H |
Disposing of PHEXXI
Step 8: Throw Away (Dispose of) the Used PHEXXI Pre-filled Applicator
- Used PHEXXI pre-filled applicators and caps should be disposed of in the trash. The cap may be a potential choking hazard.
Storing PHEXXI
- Store PHEXXI at room temperature between 68°F to 77°F (20°C to 25°C).
- Store PHEXXI in the original foil pouch.
Keep PHEXXI and all medicines out of the reach of children.
These Instructions for Use have been approved by the U.S. Food and Drug Administration.
For more information, including full prescribing information and information on patient safety, go to www.phexxi.com or call 1-833-EVFMBIO.
Manufactured for Evofem, Inc., a wholly owned subsidiary of Evofem
Biosciences, Inc.
©2023 Evofem, Inc. All rights reserved.
Issued: June 2023