Acetaminophen and Ibuprofen
Good Neighbor Pharmacy Acetaminophen and Ibuprofen Tablets
04dc36e8-4d51-aabe-e063-6294a90a0d53
HUMAN OTC DRUG LABEL
May 15, 2025
AMERISOURCE BERGEN
DUNS: 007914906
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Acetaminophen and Ibuprofen
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Drug Labeling Information
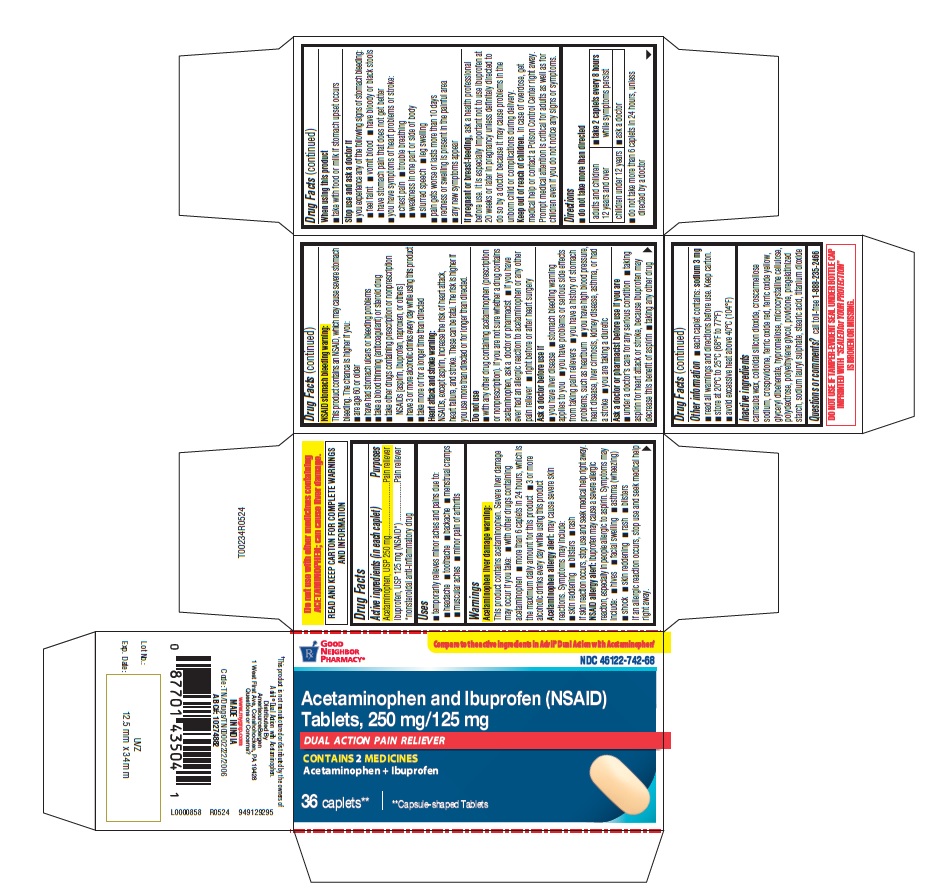
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Compare to the active ingredient in Advil® Dual Action with Acetaminophen
NDC 46122-742-68
Good
Neighbor
Pharmacy
Acetaminophen and Ibuprofen (NSAID)
Tablets, 250 mg/125 mg
DUAL ACTION PAIN RELIEVER
CONTAINS 2 MEDICINES
Acetaminophen + Ibuprofen
36 Caplets**
**capsule-shaped tablets
bottle

Carton
INDICATIONS & USAGE SECTION
Uses
- temporarily relieves minor aches and pains due to:
- headache
- toothache
- backache
- menstrual cramps
- muscular aches
- minor pain of arthritis
WARNINGS SECTION
Warnings
Acetaminophen liver damage warning:
This product contains acetaminophen. Severe liver damage may occur if you take:
- with other drugs containing acetaminophen
- more than 6 caplets in 24 hours, which is the maximum daily amount for this product
- 3 or more alcoholic drinks every day while using this product.
Acetaminophen allergy alert:
may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help right away.
**NSAID allergy alert:**ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
NSAID stomach bleeding warning:
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Heart attack and stroke warning:
NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
- if you have ever had an allergic reaction to acetaminophen or any other pain reliever
- right before or after heart surgery
Ask a doctor before use if
- you have liver disease
- stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, or had a stroke
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- under a doctor's care for any serious condition
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
When using this product
- take with food or milk if stomach upset occurs
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- weakness in one part or side of body
- slurred speech
- leg swelling
- pain gets worse or lasts more than 10 days
- redness or swelling is present in the painful area
- any new symptoms appear
**If pregnant or breast-feeding,**ask a health professional before use. It is especially important not to use ibuprofen at 20 weeks or later in pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
**Keep out of reach of children.**In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
DOSAGE & ADMINISTRATION SECTION
Directions
■ do not take more than directed
|
adults and children 12 years and over |
■take 2 caplets every 8 hourswhile symptoms persist |
|
children under 12 years |
■ ask a doctor |
■ do not take more than 6 caplets in 24 hours, unless directed by a doctor
STORAGE AND HANDLING SECTION
Other information
- each caplet contains:sodium 3 mg
- read all warnings and directions before use. Keep carton.
- store at 20°C to 25°C (68°F to 77°F)
- avoid excessive heat above 40°C (104°F)
INACTIVE INGREDIENT SECTION
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, crospovidone, ferric oxide red, ferric oxide yellow, glyceryl dibehenate, hypromellose, microcrystalline cellulose, polydextrose, polyethylene glycol, povidone, pregelatinized starch, sodium lauryl sulphate, stearic acid, titanium dioxide
OTC - QUESTIONS SECTION
Questions or comments?
call toll-free1-888-235-2466
SPL UNCLASSIFIED SECTION
***This product is not manufactured or distributed by the owners of Advil ®Dual Action with Acetaminophen.
DO NOT USE IF TAMPER-EVIDENT SEAL UNDER BOTTLE CAP IMPRINTED WITH “ SEALED for YOUR PROTECTION” IS BROKEN OR MISSING.
Do not use with other medicines containing ACETAMINOPHEN; can cause liver damage.
READ AND KEEP CARTON FOR COMPLETE WARNINGS AND INFORMATION
Distributed by:
AmerisourceBergen
1 West First Ave, Conshohocken, PA 19428
Questions or Concerns?
www.mygnp.com
MADE IN INDIA
Code: TN/Drugs/TN00002222/2006
ABC#: 10274882
R0524
L0000858
949129295
T00234R0524
Lot No.:
Exp. Date:
OTC - ACTIVE INGREDIENT SECTION
Active ingredients (in each caplet)
Acetaminophen, USP 250 mg
Ibuprofen, USP 125 mg (NSAID*)
*nonsteroidal anti-inflammatory drug
OTC - PURPOSE SECTION
Purposes
Pain reliever
Pain reliever
