Doxazosin
These highlights do not include all the information needed to use DOXAZOSIN TABLETS safely and effectively. See full prescribing information for DOXAZOSIN TABLETS. DOXAZOSIN tablets, for oral useInitial U.S. Approval: 1990
b4c974ad-cbd6-4811-ab48-461e7ffa99f7
HUMAN PRESCRIPTION DRUG LABEL
Sep 17, 2025
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Doxazosin Mesylate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
DOXAZOSIN

HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Product: 50090-6298
NDC: 50090-6298-0 30 TABLET in a BOTTLE
NDC: 50090-6298-1 100 TABLET in a BOTTLE
NDC: 50090-6298-2 90 TABLET in a BOTTLE
DESCRIPTION SECTION
11 DESCRIPTION
Doxazosin is a quinazoline compound that is a selective inhibitor of the alpha1 subtype of alpha-adrenergic receptors. The chemical name of doxazosin mesylate is 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(1,4 benzodioxan-2-ylcarbonyl) piperazine methanesulfonate. The molecular formula for doxazosin mesylate is C23H25N5O5 ∙ CH4O3S and the molecular weight is 547.6. It has the following structure:
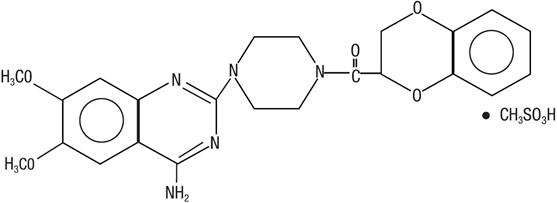
Doxazosin is freely soluble in dimethylsulfoxide, soluble in dimethylformamide, slightly soluble in methanol, ethanol, and water (0.8% at 25°C), and very slightly soluble in acetone and methylene chloride. Doxazosin tablets, USP are available as tablets for oral use and contains 1 mg, 2 mg, 4 mg and 8 mg of doxazosin as the free base.
The inactive ingredients for all tablets are: microcrystalline cellulose, anhydrous lactose, sodium starch glycolate, magnesium stearate and sodium lauryl sulfate.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Benign Prostatic Hyperplasia (BPH)
The efficacy of doxazosin was evaluated extensively in over 900 patients with BPH in double-blind, placebo-controlled trials. Doxazosin treatment was superior to placebo in improving patient symptoms and urinary flow rate. Significant relief with doxazosin was seen as early as one week into the treatment regimen, with doxazosin tablets-treated patients (N = 173) showing a significant (p<0.01) increase in maximum flow rate of 0.8 mL/sec compared to a decrease of 0.5 mL/sec in the placebo group (N = 41). In long-term studies, improvement was maintained for up to 2 years of treatment. In 66% to 71% of patients, improvements above baseline were seen in both symptoms and maximum urinary flow rate.
In three placebo-controlled studies of 14 to 16 weeks' duration, obstructive symptoms (hesitation, intermittency, dribbling, weak urinary stream, incomplete emptying of the bladder) and irritative symptoms (nocturia, daytime frequency, urgency, burning) of BPH were evaluated at each visit by patient- assessed symptom questionnaires. The bothersomeness of symptoms was measured with a modified Boyarsky questionnaire. Symptom severity/frequency was assessed using a modified Boyarsky questionnaire or an AUA-based questionnaire. Uroflowmetric evaluations were performed at times of peak (2 to 6 hours post-dose) and/or trough (24 hours post-dose) plasma concentrations of doxazosin.
The results from the three placebo-controlled studies (N = 609) showing significant efficacy with 4 mg and 8 mg doxazosin are summarized in Table 3. In all three studies, doxazosin resulted in statistically significant relief of obstructive and irritative symptoms compared to placebo. Statistically significant improvements of 2.3 mL/sec to 3.3 mL/sec in maximum flow rate were seen with doxazosin in Studies 1 and 2, compared to 0.1 mL/sec to 0.7 mL/sec with placebo.
Table 3. SUMMARY OF EFFECTIVENESS DATA IN PLACEBO-CONTROLLED TRIALS
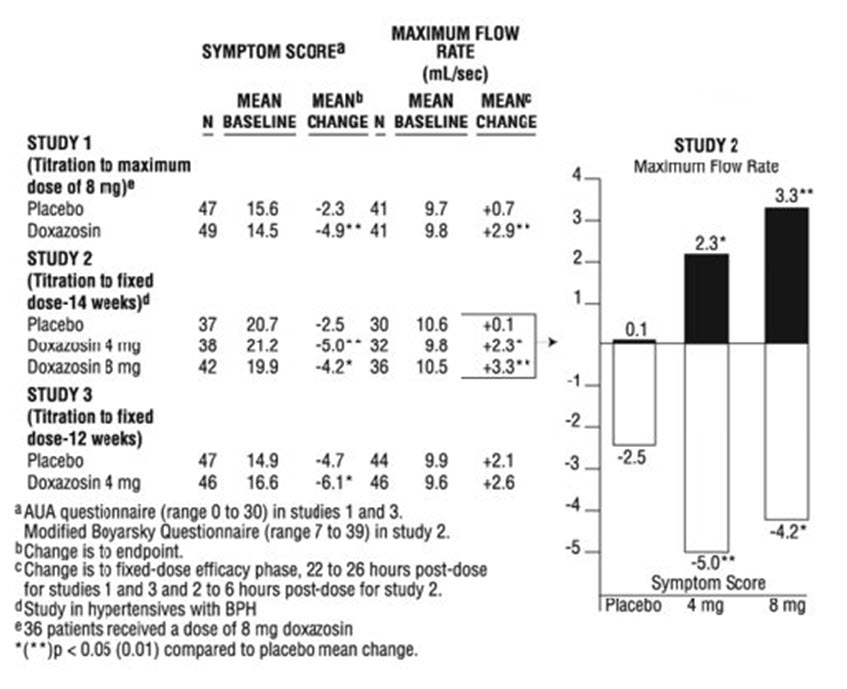
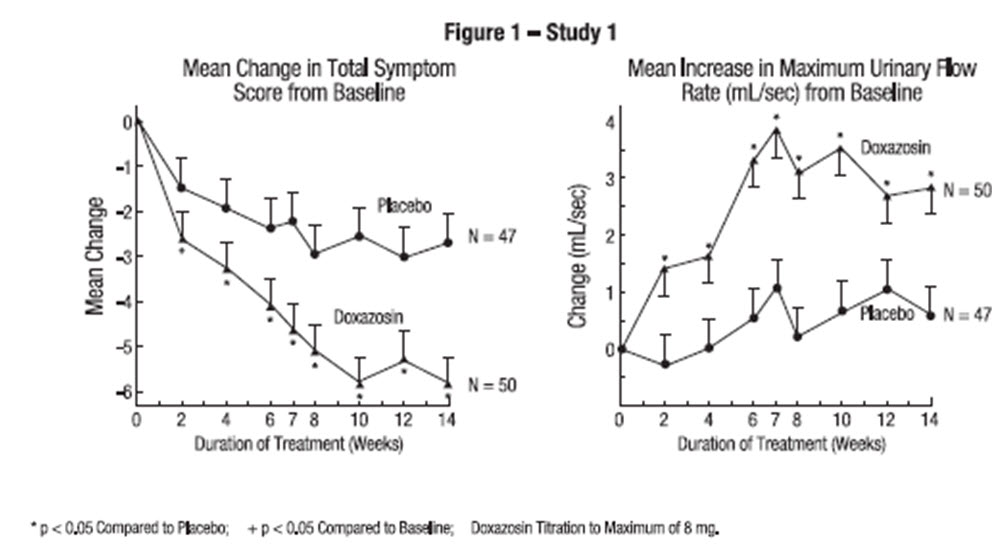
In one fixed-dose study (Study 2), doxazosin therapy (4 mg to 8 mg, once daily) resulted in a significant and sustained improvement in maximum urinary flow rate of 2.3 mL/sec to 3.3 mL/sec (Table 3) compared to placebo (0.1 mL/sec). In this study, the only study in which weekly evaluations were made, significant improvement with doxazosin versus placebo was seen after one week. The proportion of patients who responded with a maximum flow rate improvement of ≥ 3 mL/sec was significantly larger with doxazosin (34% to 42%) than placebo (13% to 17%). A significantly greater improvement was also seen in average flow rate with doxazosin (1.6 mL/sec) than with placebo (0.2 mL/sec). The onset and time course of symptom relief and increased urinary flow from Study 1 are illustrated in Figure 1.
14.2 Hypertension
In a pooled analysis of placebo-controlled hypertension studies with about 300 hypertensive patients per treatment group, doxazosin, at doses of 1 mg to 16 mg given once daily, lowered blood pressure at 24 hours by about 10/8 mmHg compared to placebo in the standing position and about 9/5 mmHg in the supine position. Peak blood pressure effects (1 to 6 hours) were larger by about 50% to 75% (i.e. trough values were about 55% to 70% of peak effect), with the larger peak-trough differences seen in systolic pressures. There was no apparent difference in the blood pressure response of Caucasians and blacks or of patients above and below age 65. In the same patient population, patients receiving doxazosin gained a mean of 0.6 kg compared to a mean loss of 0.1 kg for placebo patients.
Table 4 Mean Changes in Blood Pressure from Baseline to the Mean of the Final Efficacy Phase in Normotensives (Diastolic BP <90 mmHg) in Two Double-blind, Placebo-controlled U.S. Studies with Doxazosin 1 to 8 mg once daily.|
PLACEBO (N=85) |
Doxazosin (N=183) | |||
|---|---|---|---|---|
| ||||
|
Sitting BP (mmHg) |
Baseline |
Change |
Baseline |
Change |
|
Systolic |
128.4 |
–1.4 |
128.8 |
–4.9* |
|
Diastolic |
79.2 |
–1.2 |
79.6 |
–2.4* |
|
Standing BP (mmHg) |
Baseline |
Change |
Baseline |
Change |
|
Systolic |
128.5 |
–0.6 |
128.5 |
–5.3* |
|
Diastolic |
80.5 |
–0.7 |
80.4 |
–2.6* |
