Olanzapine
These highlights do not include all the information needed to use OLANZAPINE TABLETS safely and effectively. See full prescribing information for OLANZAPINE TABLETS. OLANZAPINE tablets, for oral use Initial U.S. Approval: 1996
a4c525da-c2f6-b804-af49-601295922254
HUMAN PRESCRIPTION DRUG LABEL
Oct 3, 2022
Dr. Reddy's Laboratories Ltd.
DUNS: 650562841
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Olanzapine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Olanzapine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
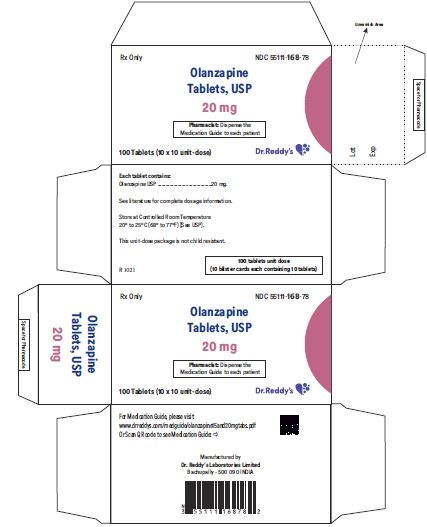
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
20 mg Carton Label:
BOXED WARNING SECTION
**WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED
PSYCHOSIS**
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
- None with olanzapine monotherapy.
- When using olanzapine and fluoxetine in combination, also refer to the Contraindications section of the package insert for Symbyax.
- For specific information about the contraindications of lithium or valproate, refer to the Contraindications section of the package inserts for these other products.
- None with olanzapine monotherapy
- When using olanzapine and fluoxetine in combination, also refer to the Contraindications section of the package insert for Symbyax®. (4)
- When using olanzapine in combination with lithium or valproate, refer to the Contraindications section of the package inserts for those products (4)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
The risks of using olanzapine in combination with other drugs have not been extensively evaluated in systematic studies.
7.1 Potential for Other Drugs to Affect Olanzapine
Diazepam — The co-administration of diazepam with olanzapine potentiated the orthostatic hypotension observed with olanzapine [seeDrug Interactions (7.2)].
Cimetidine and Antacids — Single doses of cimetidine (800 mg) or aluminum- and magnesium-containing antacids did not affect the oral bioavailability of olanzapine.
Inducers of CYP1A2 — Carbamazepine therapy (200 mg bid) causes an approximately 50% increase in the clearance of olanzapine. This increase is likely due to the fact that carbamazepine is a potent inducer of CYP1A2 activity. Higher daily doses of carbamazepine may cause an even greater increase in olanzapine clearance.
Alcohol — Ethanol (45 mg/70 kg single dose) did not have an effect on olanzapine pharmacokinetics. The co-administration of alcohol (i.e., ethanol) with olanzapine potentiated the orthostatic hypotension observed with olanzapine [seeDrug Interactions (7.2)].
Inhibitors of CYP1A2
Fluvoxamine: Fluvoxamine, a CYP1A2 inhibitor, decreases the clearance of olanzapine. This results in a mean increase in olanzapine Cmax following fluvoxamine of 54% in female nonsmokers and 77% in male smokers. The mean increase in olanzapine AUC is 52% and 108%, respectively. Lower doses of olanzapine should be considered in patients receiving concomitant treatment with fluvoxamine.
Inhibitors of CYP2D6
Fluoxetine: Fluoxetine (60 mg single dose or 60 mg daily dose for 8 days) causes a small (mean 16%) increase in the maximum concentration of olanzapine and a small (mean 16%) decrease in olanzapine clearance. The magnitude of the impact of this factor is small in comparison to the overall variability between individuals, and therefore dose modification is not routinely recommended. When using olanzapine and fluoxetine in combination, also refer to the Drug Interactions section of the package insert for Symbyax.
Warfarin — Warfarin (20 mg single dose) did not affect olanzapine pharmacokinetics [seeDrug Interactions (7.2****)].
Inducers of CYP1A2 or Glucuronyl Transferase — Omeprazole and rifampin, may cause an increase in olanzapine clearance.
Charcoal — The administration of activated charcoal (1 g) reduced the Cmax and AUC of oral olanzapine by about 60%. As peak olanzapine levels are not typically obtained until about 6 hours after dosing, charcoal may be a useful treatment for olanzapine overdose.
Anticholinergic Drugs — Concomitant treatment with olanzapine and other drugs with anticholinergic activity can increase the risk for severe gastrointestinal adverse reactions related to hypomotility. Olanzapine should be used with caution in patients receiving medications having anticholinergic (antimuscarinic) effects [see Warnings and Precautions (5.14)].
7.2 Potential for Olanzapine to Affect Other Drugs
CNS Acting Drugs — Given the primary CNS effects of olanzapine, caution should be used when olanzapine is taken in combination with other centrally acting drugs and alcohol.
Antihypertensive Agents — Olanzapine, because of its potential for inducing hypotension, may enhance the effects of certain antihypertensive agents.
Levodopa and Dopamine Agonists — Olanzapine may antagonize the effects of levodopa and dopamine agonists.
Lithium — Multiple doses of olanzapine (10 mg for 8 days) did not influence the kinetics of lithium. Therefore, concomitant olanzapine administration does not require dosage adjustment of lithium. [seeWarnings and Precautions ****(5.16)].
Valproate —Olanzapine (10 mg daily for 2 weeks) did not affect the steady state plasma concentrations of valproate. Therefore, concomitant olanzapine administration does not require dosage adjustment of valproate. [see Warnings and Precautions**(5.16)**].
Effect of Olanzapine on Drug Metabolizing Enzymes — In vitro studies utilizing human liver microsomes suggest that olanzapine has little potential to inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A. Thus, olanzapine is unlikely to cause clinically important drug interactions mediated by these enzymes.
Imipramine — Single doses of olanzapine did not affect the pharmacokinetics of imipramine or its active metabolite desipramine.
Warfarin — Single doses of olanzapine did not affect the pharmacokinetics of warfarin [seeDrug Interactions (7.1)].
Diazepam — Olanzapine did not influence the pharmacokinetics of diazepam or its active metabolite N-desmethyldiazepam. However, diazepam co-administered with olanzapine increased the orthostatic hypotension observed with either drug given alone. [seeDrug Interactions****(7.1)].
Alcohol — Multiple doses of olanzapine did not influence the kinetics of ethanol [seeDrug Interactions (7.1)].
Biperiden — Multiple doses of olanzapine did not influence the kinetics of biperiden.
Theophylline — Multiple doses of olanzapine did not affect the pharmacokinetics of theophylline or its metabolites.
- Diazepam: May potentiate orthostatic hypotension (7.1,7.2)
- Alcohol: May potentiate orthostatic hypotension (7.1)
- Carbamazepine: Increased clearance of olanzapine (7.1)
- Fluvoxamine: May increase olanzapine levels (7.1)
- Olanzapine and Fluoxetine in Combination: Also refer to the DrugInteractions section of the package insert for Symbyax. (7.1)
- CNS Acting Drugs: Caution should be used when taken in combination with other centrally acting drugs and alcohol (7.2)
- Antihypertensive Agents: Enhanced antihypertensive effect (7.2)
- Levodopa and Dopamine Agonists: May antagonize levodopa/dopamine agonists (7.2)
- Other Concomitant Drug Therapy: When using olanzapine in combination with lithium or valproate, refer to the Drug Interactions sections of the package insert for those products (7.2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Olanzapine Tablets USP, 15 mg are white, film coated, round, biconvex tablets with “R”
15
debossed on one side and “0167” on other side.
Olanzapine Tablets USP, 20 mg are white, film coated, oval, biconvex tablets with “R”
20
debossed on one side and “0168” on other side.
- Tablets (not scored): 15, 20 mg (3)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warnings and Precautions (5.11) 1/2025
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
When using olanzapine and fluoxetine in combination, also refer to the Use in Specific Populations section of the package insert for Symbyax.
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including olanzapine, during pregnancy. Healthcare providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research- programs/pregnancyregistry/.
Risk Summary
Neonates exposed to antipsychotic drugs, including olanzapine, during the third trimester are at risk for extrapyramidal and/or withdrawal symptoms following delivery (see Clinical Considerations). Overall available data from published epidemiologic studies of pregnant women exposed to olanzapine have not established a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are risks to the mother associated with untreated schizophrenia or bipolar I disorder and with exposure to antipsychotics, including olanzapine, during pregnancy (see Clinical Considerations).
Olanzapine was not teratogenic when administered orally to pregnant rats and rabbits at doses that are 9- and 30-times the daily oral maximum recommended human dose (MRHD), based on mg/m2 body surface area; some fetal toxicities were observed at these doses (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk
There is a risk to the mother from untreated schizophrenia or bipolar I disorder, including increased risk of relapse, hospitalization, and suicide. Schizophrenia and bipolar I disorder are associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
Fetal/Neonatal adverse reactions
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs, including olanzapine, during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization.
Data
Human Data
Placental passage has been reported in published study reports; however, the placental passage ratio was highly variable ranging between 7% to 167% at birth following exposure during pregnancy. The clinical relevance of this finding is unknown.
Published data from observational studies, birth registries, and case reports that have evaluated the use of atypical antipsychotics during pregnancy do not establish an increased risk of major birth defects. A retrospective cohort study from a Medicaid database of 9258 women exposed to antipsychotics during pregnancy did not indicate an overall increased risk for major birth defects.
Animal Data
In oral reproduction studies in rats at doses up to 18 mg/kg/day and in rabbits at doses up to 30 mg/kg/day (9 and 30 times the daily oral MRHD based on mg/m2 body surface area, respectively), no evidence of teratogenicity was observed. In an oral rat teratology study, early resorptions and increased numbers of nonviable fetuses were observed at a dose of 18 mg/kg/day (9 times the daily oral MRHD based on mg/m2 body surface area), and gestation was prolonged at 10 mg/kg/day (5 times the daily oral MRHD based on mg/m2body surface area). In an oral rabbit teratology study, fetal toxicity manifested as increased resorptions and decreased fetal weight, occurred at a maternally toxic dose of 30 mg/kg/day (30 times the daily oral MRHD based on mg/m2 body surface area).
8.2 Lactation
Risk Summary
Olanzapine is present in human milk. There are reports of excess sedation, irritability, poor feeding and extrapyramidal symptoms (tremors and abnormal muscle movements) in infants exposed to olanzapine through breast milk (see Clinical Considerations). There is no information on the effects of olanzapine on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for olanzapine and any potential adverse effects on the breastfed child from olanzapine or from the mother’s underlying condition.
Clinical Considerations
Infants exposed to olanzapine should be monitored for excess sedation, irritability, poor feeding, and extrapyramidal symptoms (tremors and abnormal muscle movements).
8.3 Females and Males of Reproductive Potential
Infertility
Females
Based on the pharmacologic action of olanzapine (D2 receptor antagonism), treatment with olanzapine may result in an increase in serum prolactin levels, which may lead to a reversible reduction in fertility in females of reproductive potential [seeWarnings and Precautions (5.15)].
8.4 Pediatric Use
The safety and effectiveness of oral olanzapine in the treatment of schizophrenia and manic or mixed episodes associated with bipolar I disorder were established in short-term studies in adolescents (ages 13 to 17 years). Use of olanzapine in adolescents is supported by evidence from adequate and well-controlled studies of olanzapine in which 268 adolescents received olanzapine in a range of 2.5 to 20 mg/day [seeClinical Studies (14.1,14.2)]. Recommended starting dose for adolescents is lower than that for adults [see Dosageand Administration (2.1, 2.2)]. Compared to patients from adult clinical trials, adolescents were likely to gain more weight, experience increased sedation, and have greater increases in total cholesterol, triglycerides, LDL cholesterol, prolactin and hepatic aminotransferase levels [see Warnings and Precautions (5.5, 5.15, 5.17) and Adverse Reactions**(6.1)]. When deciding among the alternative treatments available for adolescents, clinicians should consider the increased potential (in adolescents as compared with adults) for weight gain and dyslipidemia. Clinicians should consider the potential long-term risks when prescribing to adolescents, and in many cases this may lead them to consider prescribing other drugs first in adolescents [seeIndications and Usage (1.1,1.2)** ].
Safety and effectiveness of olanzapine in children <13 years of age have not been established [seePatient Counseling Information (17)].
Safety and efficacy of olanzapine and fluoxetine in combination in children and adolescents (10 to 17 years of age) have been established for the acute treatment of depressive episodes associated with bipolar I disorder.
Safety and effectiveness of olanzapine and fluoxetine in combination in children <10 years of age have not been established.
8.5 Geriatric Use
Of the 2500 patients in premarketing clinical studies with oral olanzapine, 11% (263) were 65 years of age or over. In patients with schizophrenia, there was no indication of any different tolerability of olanzapine in the elderly compared to younger patients. Studies in elderly patients with dementia- related psychosis have suggested that there may be a different tolerability profile in this population compared to younger patients with schizophrenia. Elderly patients with dementia-related psychosis treated with olanzapine are at an increased risk of death compared to placebo. In placebo-controlled studies of olanzapine in elderly patients with dementia-related psychosis, there was a higher incidence of cerebrovascular adverse events (e.g., stroke, transient ischemic attack) in patients treated with olanzapine compared to patients treated with placebo. In 5 placebo-controlled studies of olanzapine in elderly patients with dementia-related psychosis (n=1184), the following adverse reactions were reported in olanzapine-treated patients at an incidence of at least 2% and significantly greater than placebo-treated patients: falls, somnolence, peripheral edema, abnormal gait, urinary incontinence, lethargy, increased weight, asthenia, pyrexia, pneumonia, dry mouth and visual hallucinations. The rate of discontinuation due to adverse reactions was greater with olanzapine than placebo (13% vs 7%). Elderly patients with dementia-related psychosis treated with olanzapine are at an increased risk of death compared to placebo. Olanzapine is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning, Warnings and Precautions (5.1), and Patient Counseling Information (17)]. Olanzapine is not approved for the treatment of patients with dementia-related psychosis. Also, the presence of factors that might decrease pharmacokinetic clearance or increase the pharmacodynamic response to olanzapine should lead to consideration of a lower starting dose for any geriatric patient [seeBoxed Warning****,Warnings and Precautions (5.1),****and Dosage and Administration (2.1)].
Clinical studies of olanzapine and fluoxetine in combination did not include sufficient numbers of patients ≥65 years of age to determine whether they respond differently from younger patients.
- Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. (8.1)
- Pediatric Use: Safety and effectiveness of olanzapine in children <13 years of age have not been established. Safety and effectiveness of olanzapine and fluoxetine in combination in children < 10 years of age have not been established. (8.4).
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.3 Dependence
In studies prospectively designed to assess abuse and dependence potential, olanzapine was shown to have acute depressive CNS effects but little or no potential of abuse or physical dependence in rats administered oral doses up to 15 times the daily oral MRHD (20 mg) and rhesus monkeys administered oral doses up to 8 times the daily oral MRHD based on mg/m2 body surface area.
Olanzapine has not been systematically studied in humans for its potential for abuse, tolerance, or physical dependence. While the clinical trials did not reveal any tendency for any drug-seeking behavior, these observations were not systematic, and it is not possible to predict on the basis of this limited experience the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed. Consequently, patients should be evaluated carefully for a history of drug abuse, and such patients should be observed closely for signs of misuse or abuse of olanzapine (e.g., development of tolerance, increases in dose, drug-seeking behavior).
OVERDOSAGE SECTION
10 OVERDOSAGE
10.1 Human Experience
In premarketing trials involving more than 3100 patients and/or normal subjects, accidental or intentional acute overdosage of olanzapine was identified in 67 patients. In the patient taking the largest identified amount, 300 mg, the only symptoms reported were drowsiness and slurred speech. In the limited number of patients who were evaluated in hospitals, including the patient taking 300 mg, there were no observations indicating an adverse change in laboratory analytes or ECG. Vital signs were usually within normal limits following overdoses.
In postmarketing reports of overdose with olanzapine alone, symptoms have been reported in the majority of cases. In symptomatic patients, symptoms with ≥10% incidence included agitation/aggressiveness, dysarthria, tachycardia, various extrapyramidal symptoms, and reduced level of consciousness ranging from sedation to coma. Among less commonly reported symptoms were the following potentially medically serious reactions: aspiration, cardiopulmonary arrest, cardiac arrhythmias (such as supraventricular tachycardia and 1 patient experiencing sinus pause with spontaneous resumption of normal rhythm), delirium, possible neuroleptic malignant syndrome, respiratory depression/arrest, convulsion, hypertension, and hypotension. Reports of fatality in association with overdose of olanzapine alone have been received**.** In 1 case of death, the amount of acutely ingested olanzapine was reported to be possibly as low as 450 mg of oral olanzapine; however, in another case, a patient was reported to survive an acute olanzapine ingestion of approximately 2 g of oral olanzapine.
10.2 Management of Overdose
There is no specific antidote to an overdose of olanzapine. The possibility of multiple drug involvement should be considered. Establish and maintain an airway and ensure adequate oxygenation and ventilation. Cardiovascular monitoring should commence immediately and should include continuous electrocardiographic monitoring to detect possible arrhythmias.
Contact a Certified Poison Control Center for the most up to date information on the management of overdosage (1-800-222- 1222).
For specific information about overdosage with lithium or valproate, refer to the Overdosage section of the prescribing information for those products. For specific information about overdosage with olanzapine and fluoxetine in combination, refer to the Overdosage section of the Symbyax prescribing information.
DESCRIPTION SECTION
11 DESCRIPTION
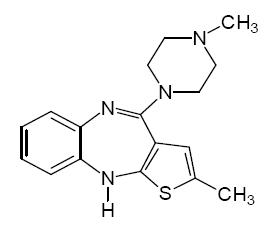
Olanzapine USP is an atypical antipsychotic that belongs to the thienobenzodiazepine class. The chemical designation is 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b] [1,5]benzodiazepine. The molecular formula is C17H20N4S, which corresponds to a molecular weight of 312.44. The chemical structure is:
Olanzapine USP is a yellow crystalline solid, which is practically insoluble in water.
Olanzapine tablets USP are intended for oral administration only.
Each tablet contains olanzapine USP equivalent to 15 mg (48 μmol) and 20 mg (64 µmol). Inactive ingredients are crospovidone, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose (Avicel PH 101) and microcrystalline cellulose (Avicel PH 102). The color coating contains hypromellose, polyethylene glycol 400 and titanium dioxide.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenesis — Oral carcinogenicity studies were conducted in mice and rats. Olanzapine was administered to mice in two 78-week studies at doses of 3, 10, 30/20 mg/kg/day (equivalent to 0.8 to 5 times the daily oral MRHD based on mg/m2 body surface area) and 0.25, 2, 8 mg/kg/day (equivalent to 0.06 to 2 times the daily oral MRHD based on mg/m2 body surface area). Rats were dosed for 2 years at doses of 0.25, 1, 2.5, 4 mg/kg/day (males) and 0.25, 1, 4, 8 mg/kg/day (females) (equivalent to 0.13-2 and 0.13-4 times the daily oral MRHD based on mg/m2 body surface area, respectively). The incidence of liver hemangiomas and hemangiosarcomas was significantly increased in 1 mouse study in female mice at 2 times the daily oral MRHD based on mg/m2 body surface area. These tumors were not increased in another mouse study in females dosed up to 2-5 times the daily oral MRHD based on mg/m2 body surface area; in this study, there was a high incidence of early mortalities in males of the 30/20 mg/kg/day group. The incidence of mammary gland adenomas and adenocarcinomas was significantly increased in female mice dosed at ≥2 mg/kg/day and in female rats dosed at ≥4 mg/kg/day (0.5 and 2 times the daily oral MRHD based on mg/m2 body surface area, respectively). Antipsychotic drugs have been shown to chronically elevate prolactin levels in rodents. Serum prolactin levels were not measured during the olanzapine carcinogenicity studies; however, measurements during subchronic toxicity studies showed that olanzapine elevated serum prolactin levels up to 4-fold in rats at the same doses used in the carcinogenicity study. An increase in mammary gland neoplasms has been found in rodents after chronic administration of other antipsychotic drugs and is considered to be prolactin mediated. The relevance for human risk of the finding of prolactin mediated endocrine tumors in rodents is unknown [see Warnings and Precautions (5.15)].
Mutagenesis — No evidence of genotoxic potential for olanzapine was found in the Ames reverse mutation test, in vivo micronucleus test in mice, the chromosomal aberration test in Chinese hamster ovary cells, unscheduled DNA synthesis test in rat hepatocytes, induction of forward mutation test in mouse lymphoma cells, or in vivo sister chromatid exchange test in bone marrow of Chinese hamsters.
Impairment of Fertility — In an oral fertility and reproductive performance study in rats, male mating performance, but not fertility, was impaired at a dose of 22.4 mg/kg/day and female fertility was decreased at a dose of 3 mg/kg/day (11 and 1.5 times the daily oral MRHD based on mg/m2 body surface area, respectively). Discontinuance of olanzapine treatment reversed the effects on male mating performance. In female rats, the precoital period was increased and the mating index reduced at 5 mg/kg/day (2.5 times the daily oral MRHD based on mg/m2 body surface area. Diestrous was prolonged and estrous delayed at 1.1 mg/kg/day (0.6 times the daily oral MRHD based on mg/m2 body surface area); therefore olanzapine may produce a delay in ovulation.
13.2 Animal Toxicology and/or Pharmacology
In animal studies with olanzapine, the principal hematologic findings were reversible peripheral cytopenias in individual dogs dosed at 10 mg/kg (17 times the daily oral MRHD based on mg/m2 body surface area), dose-related decreases in lymphocytes and neutrophils in mice, and lymphopenia in rats. A few dogs treated with 10 mg/kg developed reversible neutropenia and/or reversible hemolytic anemia between 1 and 10 months of treatment. Dose-related decreases in lymphocytes and neutrophils were seen in mice given doses of 10 mg/kg (equal to 2 times the daily oral MRHD based on mg/m2 body surface area) in studies of 3 months’ duration. Nonspecific lymphopenia, consistent with decreased body weight gain, occurred in rats receiving 22.5 mg/kg (11 times the daily oral MRHD based on mg/m2 body surface area) for 3 months or 16 mg/kg (8 times the daily oral MRHD based on mg/m2 body surface area) for 6 or 12 months. No evidence of bone marrow cytotoxicity was found in any of the species examined. Bone marrows were normocellular or hypercellular, indicating that the reductions in circulating blood cells were probably due to peripheral (non-marrow) factors.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of olanzapine, in the listed indications is unclear. However, the efficacy of olanzapine in schizophrenia could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism.
12.2 Pharmacodynamics
Olanzapine binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki=4, 11, and 5 nM, respectively), dopamine D1-4 (Ki=11-31 nM), histamine H1 (Ki=7 nM), and adrenergic α1 receptors (Ki=19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki=57 nM) and muscarinic M1-5 (Ki=73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to GABAA, BZD, and β adrenergic receptors (Ki>10 μM).
12.3 Pharmacokinetics
Oral Administration, Monotherapy — Olanzapine is well absorbed and reaches peak concentrations in approximately 6 hours following an oral dose. It is eliminated extensively by first pass metabolism, with approximately 40% of the dose metabolized before reaching the systemic circulation. Food does not affect the rate or extent of olanzapine absorption.
Olanzapine displays linear kinetics over the clinical dosing range. Its half- life ranges from 21 to 54 hours (5th to 95th percentile; mean of 30 hr), and apparent plasma clearance ranges from 12 to 47 L/hr (5th to 95th percentile; mean of 25 L/hr). Administration of olanzapine once daily leads to steady- state concentrations in about 1 week that are approximately twice the concentrations after single doses. Plasma concentrations, half-life, and clearance of olanzapine may vary between individuals on the basis of smoking status, gender, and age.
Olanzapine is extensively distributed throughout the body, with a volume of distribution of approximately 1000 L. It is 93% bound to plasma proteins over the concentration range of 7 to 1100 ng/mL, binding primarily to albumin and α1-acid glycoprotein.
Metabolism and Elimination — Following a single oral dose of 14C labeled olanzapine, 7% of the dose of olanzapine was recovered in the urine as unchanged drug, indicating that olanzapine is highly metabolized. Approximately 57% and 30% of the dose was recovered in the urine and feces, respectively. In the plasma, olanzapine accounted for only 12% of the AUC for total radioactivity, indicating significant exposure to metabolites. After multiple dosing, the major circulating metabolites were the 10-N-glucuronide, present at steady state at 44% of the concentration of olanzapine, and 4´-N-desmethyl olanzapine, present at steady state at 31% of the concentration of olanzapine. Both metabolites lack pharmacological activity at the concentrations observed. Direct glucuronidation and cytochrome P450 (CYP) mediated oxidation are the primary metabolic pathways for olanzapine. In vitro studies suggest that CYPs 1A2 and 2D6, and the flavin-containing monooxygenase system are involved in olanzapine oxidation. CYP2D6 mediated oxidation appears to be a minor metabolic pathway in vivo, because the clearance of olanzapine is not reduced in subjects who are deficient in this enzyme.
Specific Populations
Renal Impairment — Because olanzapine is highly metabolized before excretion and only 7% of the drug is excreted unchanged, renal dysfunction alone is unlikely to have a major impact on the pharmacokinetics of olanzapine. The pharmacokinetic characteristics of olanzapine were similar in patients with severe renal impairment and normal subjects, indicating that dosage adjustment based upon the degree of renal impairment is not required. In addition, olanzapine is not removed by dialysis. The effect of renal impairment on metabolite elimination has not been studied.
Hepatic Impairment — Although the presence of hepatic impairment may be expected to reduce the clearance of olanzapine, a study of the effect of impaired liver function in subjects (n=6) with clinically significant (Childs Pugh Classification A and B) cirrhosis revealed little effect on the pharmacokinetics of olanzapine.
Geriatric — In a study involving 24 healthy subjects, the mean elimination half-life of olanzapine was about 1.5 times greater in elderly (≥65 years) than in non-elderly subjects (<65 years). Caution should be used in dosing the elderly, especially if there are other factors that might additively influence drug metabolism and/or pharmacodynamic sensitivity [seeDosage and Administration (2)].
Gender — Clearance of olanzapine is approximately 30% lower in women than in men. There were, however, no apparent differences between men and women in effectiveness or adverse effects. Dosage modifications based on gender should not be needed.
Smoking Status — Olanzapine clearance is about 40% higher in smokers than in nonsmokers, although dosage modifications are not routinely recommended.
Race — In vivo studies have shown that exposures are similar among Japanese, Chinese and Caucasians, especially after normalization for body weight differences. Dosage modifications for race are, therefore, not recommended.
Combined Effects — The combined effects of age, smoking, and gender could lead to substantial pharmacokinetic differences in populations. The clearance in young smoking males, for example, may be 3 times higher than that in elderly nonsmoking females. Dosing modification may be necessary in patients who exhibit a combination of factors that may result in slower metabolism of olanzapine [seeDosage and Administration (2)].
Adolescents (ages 13 to 17 years) —In clinical studies, most adolescents were nonsmokers and this population had a lower average body weight, which resulted in higher average olanzapine exposure compared to adults.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
When using olanzapine and fluoxetine in combination, also refer to the Clinical Studies section of the package insert for Symbyax.
14.1 Schizophrenia
Adults
The efficacy of oral olanzapine in the treatment of schizophrenia was established in 2 short-term (6-week) controlled trials of adult inpatients who met DSM III-R criteria for schizophrenia. A single haloperidol arm was included as a comparative treatment in 1 of the 2 trials, but this trial did not compare these 2 drugs on the full range of clinically relevant doses for both.
Several instruments were used for assessing psychiatric signs and symptoms in these studies, among them the Brief Psychiatric Rating Scale (BPRS), a multi- item inventory of general psychopathology traditionally used to evaluate the effects of drug treatment in schizophrenia. The BPRS psychosis cluster (conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content) is considered a particularly useful subset for assessing actively psychotic schizophrenic patients. A second traditional assessment, the Clinical Global Impression (CGI), reflects the impression of a skilled observer, fully familiar with the manifestations of schizophrenia, about the overall clinical state of the patient. In addition, 2 more recently developed scales were employed; these included the 30-item Positive and Negative Symptoms Scale (PANSS), in which are embedded the 18 items of the BPRS, and the Scale for Assessing Negative Symptoms (SANS). The trial summaries below focus on the following outcomes: PANSS total and/or BPRS total; BPRS psychosis cluster; PANSS negative subscale or SANS; and CGI Severity. The results of the trials follow:
-
In a 6-week, placebo-controlled trial (n=149) involving 2 fixed olanzapine doses of 1 and 10 mg/day (once daily schedule), olanzapine, at 10 mg/day (but not at 1 mg/day), was superior toplacebo on the PANSS total score (also on the extracted BPRS total), on the BPRS psychosis cluster, on the PANSS Negative subscale, and on CGI Severity.
-
In a 6-week, placebo-controlled trial (n=253) involving 3 fixed dose ranges of olanzapine (5 ± 2.5 mg/day, 10 ± 2.5 mg/day, and 15 ± 2.5 mg/day) on a once daily schedule, the 2 highest olanzapine dose groups (actual mean doses of 12 and 16 mg/day, respectively) were superior to placebo on BPRS total score, BPRS psychosis cluster, and CGI severity score; the highest olanzapine dose group was superior to placebo on the SANS. There was no clear advantage for the high-dose group over the medium-dose group.
-
In a longer-term trial, adult outpatients (n=326) who predominantly met DSM-IV criteria for schizophrenia and who remained stable on olanzapine during open-label treatment for at least 8 weeks were randomized to continuation on their current olanzapine doses (ranging from 10 to 20 mg/day) or to placebo. The follow-up period to observe patients for relapse, defined in terms of increases in BPRS positive symptoms or hospitalization, was planned for 12 months, however, criteria were met for stopping the trial early due to an excess of placebo relapses compared to olanzapine relapses, and olanzapine was superior to placebo on time to relapse, the primary outcome for this study. Thus, olanzapine was more effective than placebo at maintaining efficacy in patients stabilized for approximately 8 weeks and followed for an observation period of up to 8 months.
Examination of population subsets (race and gender) did not reveal any differential responsiveness on the basis of these subgroupings.
Adolescents
The efficacy of oral olanzapine in the acute treatment of schizophrenia in adolescents (ages 13 to 17 years) was established in a 6-week double-blind, placebo-controlled, randomized trial of inpatients and outpatients with schizophrenia (n=107) who met diagnostic criteria according to DSM-IV-TR and confirmed by the Kiddie Schedule for Affective Disorders and schizophrenia for School Aged Children-Present and Lifetime Version (K-SADS-PL).
The primary rating instrument used for assessing psychiatric signs and symptoms in this trial was the Anchored Version of the Brief Psychiatric Rating Scale for Children (BPRS-C) total score.
In this flexible-dose trial, olanzapine 2.5 to 20 mg/day (mean modal dose 12.5 mg/day, mean dose of 11.1 mg/day) was more effective than placebo in the treatment of adolescents diagnosed with schizophrenia, as supported by the statistically significantly greater mean reduction in BPRS-C total score for patients in the olanzapine treatment group than in the placebo group.
While there is no body of evidence available to answer the question of how long the adolescent patient treated with olanzapine should be maintained, maintenance efficacy can be extrapolated from adult data along with comparisons of olanzapine pharmacokinetic parameters in adult and adolescent patients. It is generally recommended that responding patients be continued beyond the acute response, but at the lowest dose needed to maintain remission. Patients should be periodically reassessed to determine the need for maintenance treatment.
14.2 Bipolar I Disorder (Manic or Mixed Episodes)
Adults
Monotherapy — The efficacy of oral olanzapine in the treatment of manic or mixed episodes was established in 2 short-term (one 3-week and one 4-week) placebo-controlled trials in adult patients who met the DSM-IV criteria for bipolar I disorder with manic or mixed episodes. These trials included patients with or without psychotic features and with or without a rapid- cycling course.
The primary rating instrument used for assessing manic symptoms in these trials was the Young Mania Rating Scale (Y-MRS), an 11-item clinician-rated scale traditionally used to assess the degree of manic symptomatology (irritability, disruptive/aggressive behavior, sleep, elevated mood, speech, increased activity, sexual interest, language/thought disorder, thought content, appearance, and insight) in a range from 0 (no manic features) to 60 (maximum score). The primary outcome in these trials was change from baseline in the Y-MRS total score. The results of the trials follow:
(1) In one 3-week placebo-controlled trial (n=67) which involved a dose range of olanzapine (5-20 mg/day, once daily, starting at 10 mg/day), olanzapine was superior to placebo in the reduction of Y-MRS total score. In an identically designed trial conducted simultaneously with the first trial, olanzapine demonstrated a similar treatment difference, but possibly due to sample size and site variability, was not shown to be superior to placebo on this outcome.
(2) In a 4-week placebo-controlled trial (n=115) which involved a dose range of olanzapine (5 to 20 mg/day, once daily, starting at 15 mg/day), olanzapine was superior to placebo in the reduction of Y-MRS total score.
(3) In another trial, 361 patients meeting DSM-IV criteria for a manic or mixed episode of bipolar I disorder who had responded during an initial open- label treatment phase for about 2 weeks, on average, to olanzapine 5 to 20 mg/day were randomized to either continuation of olanzapine at their same dose (n=225) or to placebo (n=136), for observation of relapse. Approximately 50% of the patients had discontinued from the olanzapine group by day 59 and 50% of the placebo group had discontinued by day 23 of double-blind treatment. Response during the open-label phase was defined by having a decrease of the Y-MRS total score to ≤12 and HAM-D 21 to ≤8. Relapse during the double-blind phase was defined as an increase of the Y-MRS or HAM-D 21 total score to ≥15, or being hospitalized for either mania or depression. In the randomized phase, patients receiving continued olanzapine experienced a significantly longer time to relapse.
Adjunct to Lithium or Valproate — The efficacy of oral olanzapine with concomitant lithium or valproate in the treatment of acute manic or mixed episodes was established in 2 controlled trials in patients who met the DSM-IV criteria for bipolar I disorder with manic or mixed episodes. These trials included patients with or without psychotic features and with or without a rapid-cycling course. The results of the trials follow:
(1) In one 6-week placebo-controlled combination trial, 175 outpatients on lithium or valproate therapy with inadequately controlled manic or mixed symptoms (Y-MRS ≥16) were randomized to receive either olanzapine or placebo, in combination with their original therapy. Olanzapine (in a dose range of 5 to 20 mg/day, once daily, starting at 10 mg/day) combined with lithium or valproate (in a therapeutic range of 0.6 mEq/L to 1.2 mEq/L or 50 mcg/ mL to 125 mcg/mL, respectively) was superior to lithium or valproate alone in the reduction of Y-MRS total score.
(2) In a second 6-week placebo-controlled combination trial, 169 outpatients on lithium or valproate therapy with inadequately controlled manic or mixed symptoms (Y-MRS ≥16) were randomized to receive either olanzapine or placebo, in combination with their original therapy. Olanzapine (in a dose range of 5 to 20 mg/day, once daily, starting at 10 mg/day) combined with lithium or valproate (in a therapeutic range of 0.6 mEq/L to 1.2 mEq/L or 50 mcg/mL to 125 mcg/mL, respectively) was superior to lithium or valproate alone in the reduction of Y-MRS total score.
Adolescents
Acute Monotherapy — The efficacy of oral olanzapine in the treatment of acute manic or mixed episodes in adolescents (ages 13 to 17 years) was established in a 3-week, double-blind, placebo-controlled, randomized trial of adolescent inpatients and outpatients who met the diagnostic criteria for manic or mixed episodes associated with bipolar I disorder (with or without psychotic features) according to the DSM-IV-TR (n=161). Diagnosis was confirmed by the K-SADS-PL.
The primary rating instrument used for assessing manic symptoms in this trial was the Adolescent Structured Young-Mania Rating Scale (Y-MRS) total score.
In this flexible-dose trial, olanzapine 2.5 to 20 mg/day (mean modal dose 10.7 mg/day, mean dose of 8.9 mg/day) was more effective than placebo in the treatment of adolescents with manic or mixed episodes associated with bipolar I disorder, as supported by the statistically significantly greater mean reduction in Y-MRS total score for patients in the olanzapine treatment group than in the placebo group.
While there is no body of evidence available to answer the question of how long the adolescent patient treated with olanzapine should be maintained, maintenance efficacy can be extrapolated from adult data along with comparisons of olanzapine pharmacokinetic parameters in adult and adolescent patients. It is generally recommended that responding patients be continued beyond the acute response, but at the lowest dose needed to maintain remission. Patients should be periodically reassessed to determine the need for maintenance treatment.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Olanzapine Tablets USP, 15 mg are white, film coated, round, biconvex tablets with “R” debossed
15
on one side and “0167” on other side and are supplied in bottles of 30, 100, 500 and unit-dose packages of 100 (10 x 10).
Bottles of 30 NDC 55111-167-30
Bottles of 100 NDC 55111-167-01
Bottles of 500 NDC 55111-167-05
Unit dose packages of 100 (10 x10) NDC 55111-167-78
Olanzapine Tablets USP, 20 mg are white, film coated, oval, biconvex tablets with “R” debossed on
20
one side and “0168” on other side and are supplied in bottles of 30, 60, 100, 500 and unit-dose packages of 100 (10 x 10).
Bottles of 30 NDC 55111-168-30
Bottles of 60 NDC 55111-168-60
Bottles of 100 NDC 55111-168-01
Bottles of 500 NDC 55111-168-05
Unit dose packages of 100 (10 x10) NDC 55111-168-78
16.2 Storage and Handling
Store olanzapine tablets at controlled room temperature, 20° to 25°C (68° to 77°F) [see USP]. The USP defines controlled room temperature as a temperature maintained thermostatically that encompasses the usual and customary working environment of 20° to 25°C (68° to 77°F); that results in a mean kinetic temperature calculated to be not more than 25°C; and that allows for excursions between 15° and 30°C (59° and 86°F) that are experienced in pharmacies, hospitals, and warehouses.
Protect olanzapine tablets from light and moisture.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) for the oral formulations.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking olanzapine as monotherapy or in combination with fluoxetine. If you do not think you are getting better or have any concerns about your condition while taking olanzapine, call your doctor. When using olanzapine and fluoxetine in combination, also refer to the Patient Counseling Information section of the package insert for Symbyax.
Elderly Patients with Dementia-Related Psychosis: Increased Mortality and Cerebrovascular Adverse Events (CVAE), Including Stroke
Patients and caregivers should be advised that elderly patients with dementia- related psychosis treated with antipsychotic drugs are at an increased risk of death. Patients and caregivers should be advised that elderly patients with dementia-related psychosis treated with olanzapine had a significantly higher incidence of cerebrovascular adverse events (e.g., stroke, transient ischemic attack) compared with placebo.
Olanzapine is not approved for elderly patients with dementia-related psychosis [seeBoxed Warning and Warnings and Precautions (5.1)].
Neuroleptic Malignant Syndrome (NMS)
Patients and caregivers should be counseled that a potentially fatal symptom complex sometimes referred to as NMS has been reported in association with administration of antipsychotic drugs, including olanzapine. Signs and symptoms of NMS include hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia) [seeWarnings and Precautions (5.3)].
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Patients should be advised to report to their health care provider at the earliest onset of any signs and symptoms that may be associated with Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [seeWarnings and Precautions (5.4)].
Hyperglycemia and Diabetes Mellitus
Patients should be advised of the potential risk of hyperglycemia-related adverse reactions. Patients should be monitored regularly for worsening of glucose control. Patients who have diabetes should follow their doctor’s instructions about how often to check their blood sugar while taking olanzapine [seeWarnings and Precautions (5.5)].
Dyslipidemia
Patients should be counseled that dyslipidemia has occurred during treatment with olanzapine. Patients should have their lipid profile monitored regularly [seeWarnings and Precautions (5.5)].
Weight Gain
Patients should be counseled that weight gain has occurred during treatment with olanzapine. Patients should have their weight monitored regularly [see Warnings and Precautions (5.5)].
Orthostatic Hypotension
Patients should be advised of the risk of orthostatic hypotension, especially during the period of initial dose titration and in association with the use of concomitant drugs that may potentiate the orthostatic effect of olanzapine, e.g., diazepam or alcohol [seeWarnings and Precautions (5.7) and Drug Interactions (7)]. Patients should be advised to change positions carefully to help prevent orthostatic hypotension, and to lie down if they feel dizzy or faint, until they feel better. Patients should be advised to call their doctor if they experience any of the following signs and symptoms associated with orthostatic hypotension: dizziness, fast or slow heart beat, or fainting.
Potential for Cognitive and Motor Impairment
Because olanzapine has the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that olanzapine therapy does not affect them adversely [seeWarnings and Precautions (5.12)].
Body Temperature RegulationPatients should be advised regarding appropriate care in avoiding overheating and dehydration. Patients should be advised to call their doctor right away if they become severely ill and have some or all of these symptoms of dehydration: sweating too much or not at all, dry mouth, feeling very hot, feeling thirsty, not able to produce urine [see **Warnings and Precautions (**5.13)].
Concomitant Medication
Patients should be advised to inform their healthcare providers if they are taking, or plan to take, Symbyax® (olanzapine and fluoxetine hydrochloride). Patients should also be advised to inform their healthcare providers if they are taking, plan to take, or have stopped taking any prescription or over-the- counter drugs, including herbal supplements, since there is a potential for interactions [seeDrug Interactions (7)].
AlcoholPatients should be advised to avoid alcohol while taking olanzapine [seeDrug Interactions (7)].
Use in Specific Populations
Pregnancy — Advise women to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with olanzapine. Advise patients that olanzapine may cause extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder) in a neonate. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to olanzapine during pregnancy [seeUse in Specific Populations (8.1)].
Lactation — Advise breastfeeding women using olanzapine to monitor infants for excess sedation, irritability, poor feeding and extrapyramidal symptoms (tremors and abnormal muscle movements) and to seek medical care if they notice these signs. [seeUse in Specific Populations (8.2)].
Infertility — Advise females of reproductive potential that olanzapine may impair fertility due to an increase in serum prolactin levels. The effects on fertility are reversible [seeUse in Specific Populations (8.3)].
Pediatric Use — Olanzapine is indicated for treatment of schizophrenia and manic or mixed episodes associated with bipolar I disorder in adolescents 13 to 17 years of age. Compared to patients from adult clinical trials, adolescents were likely to gain more weight, experience increased sedation, and have greater increases in total cholesterol, triglycerides, LDL cholesterol, prolactin, and hepatic aminotransferase levels. Patients should be counseled about the potential long-term risks associated with olanzapine and advised that these risks may lead them to consider other drugs first [see Indications and Usage (1.1,1.2)]. Safety and effectiveness of olanzapine in patients under 13 years of age have not been established. Safety and efficacy of olanzapine and fluoxetine in combination in patients 10 to 17 years of age have been established for the acute treatment of depressive episodes associated with bipolar I disorder. Safety and effectiveness of olanzapine and fluoxetine in combination in patients < 10 years of age have not been established [seeWarnings and Precautions (5.5) andUse in Specific Populations (8.4)].
Need for Comprehensive Treatment Program in Pediatric Patients
Olanzapine is indicated as an integral part of a total treatment program for pediatric patients with schizophrenia and bipolar disorder that may include other measures (psychological, educational, social) for patients with the disorder. Effectiveness and safety of olanzapine have not been established in pediatric patients less than 13 years of age. Atypical antipsychotics are not intended for use in the pediatric patient who exhibits symptoms secondary to environmental factors and/or other primary psychiatric disorders. Appropriate educational placement is essential and psychosocial intervention is often helpful. The decision to prescribe atypical antipsychotic medication will depend upon the healthcare provider’s assessment of the chronicity and severity of the patient’s symptoms [seeIndications and Usage (1.3)]****.
Symbyax® (olanzapine and fluoxetine hydrochloride) is a trademark of Eli Lilly company.
Rx Only
Manufactured by:
Dr. Reddy’s Laboratories Limited
Bachupally – 500 090 INDIA
Revised: 03/2025
SPL MEDGUIDE SECTION
Dispense with Medication Guide available at:
www.drreddys.com/medguide/olanzapine15and20mgtabs.pdf
Medication Guide
Olanzapine Tablets USP
(oh lan' za peen)
Read the Medication Guide that comes with olanzapine tablets before you start taking them and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about your medical condition or treatment. Talk with your doctor or pharmacist if there is something you do not understand or you want to learn more about olanzapine tablets.
What is the most important information I should know about olanzapine tablets?
Olanzapine tablets may cause serious side effects, including:
1. Increased risk of death in elderly people who are confused, have memory loss and have lost touch with reality (dementia-related psychosis).
2. High blood sugar (hyperglycemia).
3. High fat levels in your blood (increased cholesterol and triglycerides), especially in teenagers age 13 to 17, or when used in combination with fluoxetine in children age 10 to 17.
4. Weight gain, especially in teenagers age 13 to 17, or when used in combination with fluoxetine in children age 10 to 17.
These serious side effects are described below.
1. Increased risk of death in elderly people who are confused, have memory loss and have lost touch with reality (dementia-related psychosis). Olanzapine tablets are not approved for treating psychosis in elderly people with dementia.
2. High blood sugar (hyperglycemia). High blood sugar can happen if you have diabetes already or if you have never had diabetes. High blood sugar could lead to:
• a build up of acid in your blood due to ketones (ketoacidosis)
• coma
• death
Your doctor should do tests to check your blood sugar before you start taking olanzapine tablets and during treatment. In people who do not have diabetes, sometimes high blood sugar goes away when olanzapine tablets are stopped. People with diabetes and some people who did not have diabetes before taking olanzapine tablets need to take medicine for high blood sugar even after they stop taking olanzapine tablets.
If you have diabetes, follow your doctor’s instructions about how often to check your blood sugar while taking olanzapine tablets.
** Call your doctor** if you have any of these symptoms of high blood sugar (hyperglycemia) while taking olanzapine tablets:
• feel very thirsty
• need to urinate more than usual
• feel very hungry
• feel weak or tired
• feel sick to your stomach
• feel confused or your breath smells fruity
3. High fat levels in your blood (cholesterol and triglycerides). High fat levels may happen in people treated with olanzapine tablets, especially in teenagers (13 to 17 years old), or when used in combination with fluoxetine in children (10 to 17 years old). You may not have any symptoms, so your doctor should do blood tests to check your cholesterol and triglyceride levels before you start taking olanzapine tablets and during treatment.
** 4. Weight gain.** Weight gain is very common in people who take olanzapine tablets. Teenagers (13 to 17 years old) are more likely to gain weight and to gain more weight than adults. Children (10 to 17 years old) are also more likely to gain weight and to gain more weight than adults when olanzapine is used in combination with fluoxetine. Some people may gain a lot of weight while taking olanzapine tablets, so you and your doctor should check your weight regularly. Talk to your doctor about ways to control weight gain, such as eating a healthy, balanced diet, and exercising.
What are Olanzapine tablets?
Olanzapine tablets are prescription medicine used to treat:
• schizophrenia in people age 13 or older.
• bipolar disorder, including:
• manic or mixed episodes that happen with bipolar I disorder in people age 13 or older.
• manic or mixed episodes that happen with bipolar I disorder, when used with the medicine lithium or valproate, in adults.
• long-term treatment of bipolar I disorder in adults.
• episodes of depression that happen with bipolar I disorder, when used with the medicine fluoxetine (Prozac®), in people age 10 or older.
• Episodes of depression that do not get better after 2 other medicines, also called treatment resistant depression, when used with the medicine fluoxetine (Prozac), in adults.
Olanzapine tablets have not been approved for use in children under 13 years of age. Olanzapine in combination with fluoxetine has not been approved for use in children under 10 years of age.
The symptoms of schizophrenia include hearing voices, seeing things that are not there, having beliefs that are not true, and being suspicious or withdrawn.
The symptoms of bipolar I disorder include alternating periods of depression and high or irritable mood, increased activity and restlessness, racing thoughts, talking fast, impulsive behavior, and a decreased need for sleep.
The symptoms of treatment resistant depression include decreased mood, decreased interest, increased guilty feelings, decreased energy, decreased concentration, changes in appetite, and suicidal thoughts or behavior.
Some of your symptoms may improve with treatment. If you do not think you are getting better, call your doctor.
What should I tell my doctor before taking olanzapinetablets?
Olanzapine tablets may not be right for you. Before starting olanzapine tablets, tell your doctor if you have or had:
• heart problems
• seizures
• diabetes or high blood sugar levels (hyperglycemia)
• high cholesterol or triglyceride levels in your blood
• liver problems
• low or high blood pressure
• strokes or “mini-strokes” also called transient ischemic attacks (TIAs)
• Alzheimer’s disease
• narrow-angle glaucoma
• enlarged prostate in men
• bowel obstruction
• breast cancer
• thoughts of suicide or hurting yourself
• any other medical condition
• are pregnant or plan to become pregnant. It is not known if olanzapine tablets will harm your unborn baby.
• If you become pregnant while receiving olanzapine tablets, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or go to http://womensmentalhealth.org/clinical-andresearch- programs/pregnancyregistry/.
• are breast-feeding or plan to breast-feed. Olanzapine passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take olanzapine tablets.
Tell your doctor if you exercise a lot or are in hot places often.
The symptoms of bipolar I disorder or schizophrenia may includethoughts of suicideor of hurting yourself or others. If you have these thoughts at any time, tell your doctor or go to an emergency room right away.
**Tell your doctor about all the medicines that you take,**including prescription and non-prescription medicines, vitamins, and herbal supplements. Olanzapine tablets and some medicines may interact with each other and may not work as well, or cause possible serious side effects. Your doctor can tell you if it is safe to take olanzapine tablets with your other medicines. Do not start or stop any medicine while taking olanzapine tablets without talking to your doctor first.
How should I take olanzapinetablets?
• Take olanzapine tablets exactly as prescribed. Your doctor may need to change (adjust) the dose of olanzapine tablets until it is right for you.
• If you miss a dose of olanzapine tablets, take the missed dose as soon as you remember. If it is almost time for the next dose, just skip the missed dose and take your next dose at the regular time. Do not take two doses of olanzapine tablets at the same time.
•** To prevent serious side effects, do not stop taking olanzapine tablets suddenly. If you need to stop taking olanzapinetablets, your doctor can tell you how to safely stop taking it.**
•If you take too much olanzapinetablets, call your doctor or poison control center at 1-800-222-1222 right away, or get emergency treatment.
• Olanzapine tablets can be taken with or without food.
• Olanzapine tablets are usually taken one time each day
• Call your doctor if you do not think you are getting better or have any concerns about your condition while taking olanzapine tablets, .
What should I avoid while taking olanzapine tablets?
• Olanzapine tablets can cause sleepiness and may affect your ability to make decisions, think clearly, or react quickly. You should not drive, operate heavy machinery, or do other dangerous activities until you know how olanzapine tablets affects you.
• Avoid drinking alcohol while taking olanzapine tablets. Drinking alcohol while you take olanzapine tablets may make you sleepier than if you take olanzapine tablets alone.
What are the possible side effects of olanzapinetablets?
**Serious side effects may happen when you take olanzapine tablets, including: **
• See “What is the most important information I should know about olanzapine tablets?”, which describes the increased risk of death in elderly people with dementia-related psychosis and the risks of high blood sugar, high cholesterol and triglyceride levels, and weight gain.
• Increased incidence of stroke or “mini-strokes” called transient ischemic attacks (TIAs) in elderly people with dementia-related psychosis (elderly people who have lost touch with reality due to confusion and memory loss). Olanzapine tablets are not approved for these patients.
• Neuroleptic Malignant Syndrome (NMS): NMS is a rare but very serious condition that can happen in people who take antipsychotic medicines, including olanzapine tablets. NMS can cause death and must be treated in a hospital. Call your doctor right away if you become severely ill and have any of these symptoms:
• high fever
• excessive sweating
• rigid muscles
• confusion
• changes in your breathing, heartbeat, and blood pressure
•Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): DRESS can occur with olanzapine. Features of DRESS may include rash, fever, swollen glands and other internal organ involvement such as liver, kidney, lung and heart. DRESS is sometimes fatal; therefore, tell your doctor immediately if you experience any of these signs.
• Tardive Dyskinesia: This condition causes body movements that keep happening and that you can not control. These movements usually affect the face and tongue. Tardive dyskinesia may not go away, even if you stop taking olanzapine tablets. It may also start after you stop taking olanzapine tablets. Tell your doctor if you get any body movements that you can not control.
• Decreased blood pressure when you change positions, with symptoms of dizziness, fast or slow heartbeat, or fainting.
• Difficulty swallowing, that can cause food or liquid to get into your lungs.
• Seizures : Tell your doctor if you have a seizure during treatment with olanzapine tablets.
**• Problems with control of body temperature:**You could become very hot, for instance when you exercise a lot or stay in an area that is very hot. It is important for you to drink water to avoid dehydration. Call your doctor right away if you become severely ill and have any of these symptoms of dehydration:
• sweating too much or not at all
• dry mouth
• feeling very hot
• feeling thirsty
• not able to produce urine
** Common side effects of olanzapine tablets include:**lack of energy, dry mouth, increased appetite, sleepiness, tremor (shakes), having hard or infrequent stools, dizziness, changes in behavior, or restlessness.
Other common side effects in teenagers (13 to 17 years old) include: headache, stomach-area (abdominal) pain, pain in your arms or legs, or tiredness. Teenagers experienced greater increases in prolactin, liver enzymes, and sleepiness, as compared with adults.
Tell your doctor about any side effect that bothers you or that does not go away.
These are not all the possible side effects with olanzapine tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store olanzapinetablets?
• Store olanzapine tablets at room temperature, between 68°F to 77°F (20°C to 25°C).
• Keep olanzapine tablets away from light.
• Keep olanzapine tablets dry and away from moisture.
Keep olanzapine tablets and all medicines out of the reach of children.
General information about olanzapinetablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use olanzapine tablets for a condition for which it was not prescribed. Do not give olanzapine tablets to other people, even if they have the same condition. It may harm them.
This Medication Guide summarizes the most important information about olanzapine tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about olanzapine tablets that was written for healthcare professionals.
For more information about olanzapine tablets call 1-888-375-3784.
What are the ingredients in olanzapine tablets?
Active ingredient: olanzapine
Inactive ingredients: Tablets — crospovidone, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose (Avicel PH 101) and microcrystalline cellulose (Avicel PH 102). The color coating contains hypromellose, polyethylene glycol 400 and titanium dioxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Rx Only
Manufactured by:
Dr. Reddy’s Laboratories Limited
Bachupally - 500 090 INDIA
Revised: 03/2025
