Divalproex Sodium
These highlights do not include all the information needed to use DIVALPROEX SODIUM DELAYED-RELEASE TABLETS safely and effectively. See full prescribing information for DIVALPROEX SODIUM DELAYED-RELEASE TABLETS.DIVALPROEX SODIUM delayed-release tablets, for oral useInitial U.S. Approval: 1983
d67af03a-5261-4358-8787-2e729267ff7e
HUMAN PRESCRIPTION DRUG LABEL
Sep 10, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Divalproex Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (25)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Divalproex Sodium Delayed-Release 250mg Tablets

BOXED WARNING SECTION
WARNING: LIFE THREATENING ADVERSE REACTIONS
- Most common adverse reactions (reported >5%) are abdominal pain, accidental injury, alopecia, amblyopia/blurred vision, amnesia, anorexia, asthenia, ataxia, back pain, bronchitis, constipation, depression, diarrhea, diplopia, dizziness, dyspepsia, dyspnea, ecchymosis, emotional lability, fever, flu syndrome, headache, increased appetite, infection, insomnia, nausea, nervousness, nystagmus, peripheral edema, pharyngitis, rash, rhinitis, somnolence, thinking abnormal, thrombocytopenia, tinnitus, tremor, vomiting, weight gain, weight loss (6.1,6.2, 6.3).
- The safety and tolerability of valproate in pediatric patients were shown to be comparable to those in adults (8.4).
To report SUSPECTED ADVERSE REACTIONS, contact Unichem Pharmaceuticals (USA), Inc., at 1-866-562-4616 or FDA at 1-800-FDA-1088 or ww.fda.gov/medwatch
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Mania
Divalproex sodium delayed-release tablets are valproate and is indicated for the treatment of the manic episodes associated with bipolar disorder. A manic episode is a distinct period of abnormally and persistently elevated, expansive, or irritable mood. Typical symptoms of mania include pressure of speech, motor hyperactivity, reduced need for sleep, flight of ideas, grandiosity, poor judgment, aggressiveness, and possible hostility.
The efficacy of divalproex sodium delayed-release tablets was established in 3-week trials with patients meeting DSM-III-R criteria for bipolar disorder who were hospitalized for acute mania [see Clinical Studies (14.1)].
The safety and effectiveness of divalproex sodium delayed-release tablets for long-term use in mania, i.e., more than 3 weeks, has not been demonstrated in controlled clinical trials. Therefore, healthcare providers who elect to use divalproex sodium delayed-release tablets for extended periods should continually reevaluate the long-term usefulness of the drug for the individual patient.
1.2 Epilepsy
Divalproex sodium delayed-release tablets are indicated as monotherapy and adjunctive therapy in the treatment of patients with complex partial seizures that occur either in isolation or in association with other types of seizures. Divalproex sodium delayed-release tablets are also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures, and adjunctively in patients with multiple seizure types that include absence seizures.
Simple absence is defined as very brief clouding of the sensorium or loss of consciousness accompanied by certain generalized epileptic discharges without other detectable clinical signs. Complex absence is the term used when other signs are also present.
1.3 Migraine
Divalproex sodium delayed-release tablets are indicated for prophylaxis of migraine headaches. There is no evidence that divalproex sodium delayed- release tablets are useful in the acute treatment of migraine headaches.
1.4 Important Limitations
Because of the risk to the fetus of decreased IQ, neurodevelopmental disorders, neural tube defects, and other major congenital malformations, which may occur very early in pregnancy, valproate should not be used to treat women with epilepsy or bipolar disorder who are pregnant or who plan to become pregnant unless other medications have failed to provide adequate symptom control or are otherwise unacceptable.
Valproate should not be administered to a woman of childbearing potential unless other medications have failed to provide adequate symptom control or are otherwise unacceptable [see Warnings and Precautions (5.2, 5.3, 5.4), Use in Specific Populations (8.1), and Patient Counseling Information (17)].
For prophylaxis of migraine headaches, divalproex sodium is contraindicated in women who are pregnant and in women of childbearing potential who are not using effective contraception [see Contraindications (4)].
Divalproex sodium is an anti-epileptic drug indicated for:
- Treatment of manic episodes associated with bipolar disorder (1.1)
- Monotherapy and adjunctive therapy of complex partial seizures and simple and complex absence seizures; adjunctive therapy in patients with multiple seizure types that include absence seizures (1.2)
- Prophylaxis of migraine headaches (1.3)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
- Divalproex sodium delayed-release tablets should not be administered to patients with hepatic disease or significant hepatic dysfunction [see Warnings and Precautions (5.1)].
- Divalproex sodium delayed-release tablets are contraindicated in patients known to have mitochondrial disorders caused by mutations in mitochondrial DNA polymerase γ (POLG; e.g., Alpers-Huttenlocher Syndrome) and children under two years of age who are suspected of having a POLG-related disorder [see Warnings and Precautions (5.1)].
- Divalproex sodium delayed-release tablets are contraindicated in patients with known hypersensitivity to the drug [see Warnings and Precautions (5.12)].
- Divalproex sodium delayed-release tablets are contraindicated in patients with known urea cycle disorders [see Warnings and Precautions (5.6)].
- For use in prophylaxis of migraine headaches: Divalproex sodium delayed-release tablets are contraindicated in women who are pregnant and in women of childbearing potential who are not using effective contraception [see Warnings and Precautions (5.2, 5.3, 5.4) and Use in Specific Populations (8.1)].
- Hepatic disease or significant hepatic dysfunction (4, 5.1)
- Known mitochondrial disorders caused by mutations in mitochondrial DNA polymerase γ (POLG) (4, 5.1)
- Suspected POLG-related disorder in children under two years of age (4,5.1)
- Known hypersensitivity to the drug (4, 5.12)
- Urea cycle disorders (4, 5.6)
- Prophylaxis of migraine headaches: Pregnant women, women of childbearing potential not using effective contraception (4, 8.1)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Hepatic failure [see Warnings and Precautions (5.1)]
- Birth defects [see Warnings and Precautions (5.2)]
- Decreased IQ following in utero exposure [see Warnings and Precautions (5.3)]
- Pancreatitis [see Warnings and Precautions (5.5)]
- Hyperammonemic encephalopathy [see Warnings and Precautions (5.6,5.9, 5.10)]
- Suicidal behavior and ideation [see Warnings and Precautions (5.7)]
- Bleeding and other hematopoietic disorders [see Warnings and Precautions (5.8)]
- Hypothermia [see Warnings and Precautions (5.11)]
- Drug Reaction with Eosinophilia and Systemic Symptom (DRESS)/Multiorgan hypersensitivity reactions [see Warnings and Precautions (5.12)]
- Somnolence in the elderly [see Warnings and Precautions (5.14)]
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
6.1 Mania
The incidence of treatment-emergent events has been ascertained based on combined data from two three week placebo-controlled clinical trials of divalproex sodium in the treatment of manic episodes associated with bipolar disorder. The adverse reactions were usually mild or moderate in intensity, but sometimes were serious enough to interrupt treatment. In clinical trials, the rates of premature termination due to intolerance were not statistically different between placebo, divalproex sodium, and lithium carbonate. A total of 4%, 8% and 11% of patients discontinued therapy due to intolerance in the placebo, divalproex sodium, and lithium carbonate groups, respectively.
Table 2 summarizes those adverse reactions reported for patients in these trials where the incidence rate in the divalproex sodium-treated group was greater than 5% and greater than the placebo incidence, or where the incidence in the divalproex sodium-treated group was statistically significantly greater than the placebo group. Vomiting was the only reaction that was reported by significantly (p ≤ 0.05) more patients receiving divalproex sodium compared to placebo.
Table 2. Adverse Reactions Reported by > 5% of Divalproex Sodium -Treated Patients During Placebo-Controlled Trials of Acute Mania1|
1The following adverse reactions occurred at an equal or greater incidence for placebo than for divalproex sodium: back pain, headache, constipation, diarrhea, tremor, and pharyngitis. | ||
|
** Adverse Reaction** |
** Divalproex sodium** |
** Placebo** |
|
Nausea |
22 |
15 |
|
Somnolence |
19 |
12 |
|
Dizziness |
12 |
4 |
|
Vomiting |
12 |
3 |
|
Accidental Injury |
11 |
5 |
|
Asthenia |
10 |
7 |
|
Abdominal Pain |
9 |
8 |
|
Dyspepsia |
9 |
8 |
|
Rash |
6 |
3 |
The following additional adverse reactions were reported by greater than 1% but not more than 5% of the 89 divalproex sodium-treated patients in controlled clinical trials:
Body as a Whole: Chest pain, chills, chills and fever, fever, neck pain, neck rigidity.
Cardiovascular System: Hypertension, hypotension, palpitations, postural hypotension, tachycardia, vasodilation.
Digestive System: Anorexia, fecal incontinence, flatulence, gastroenteritis, glossitis, periodontal abscess.
Hemic and Lymphatic System: Ecchymosis.
Metabolic and Nutritional Disorders: Edema, peripheral edema.
Musculoskeletal System: Arthralgia, arthrosis, leg cramps, twitching.
Nervous System: Abnormal dreams, abnormal gait, agitation, ataxia, catatonic reaction, confusion, depression, diplopia, dysarthria, hallucinations, hypertonia, hypokinesia, insomnia, paresthesia, reflexes increased, tardive dyskinesia, thinking abnormalities, vertigo.
Respiratory System: Dyspnea, rhinitis.
Skin and Appendages: Alopecia, discoid lupus erythematosus, dry skin, furunculosis, maculopapular rash, seborrhea.
Special Senses: Amblyopia, conjunctivitis, deafness, dry eyes, ear pain, eye pain, tinnitus.
Urogenital System: Dysmenorrhea, dysuria, urinary incontinence.
6.2 Epilepsy
Based on a placebo-controlled trial of adjunctive therapy for treatment of complex partial seizures, divalproex sodium was generally well tolerated with most adverse reactions rated as mild to moderate in severity. Intolerance was the primary reason for discontinuation in the divalproex sodium-treated patients (6%), compared to 1% of placebo-treated patients.
Table 3 lists treatment-emergent adverse reactions which were reported by ≥ 5% of divalproex sodium-treated patients and for which the incidence was greater than in the placebo group, in the placebo-controlled trial of adjunctive therapy for treatment of complex partial seizures. Since patients were also treated with other antiepilepsy drugs, it is not possible, in most cases, to determine whether the following adverse reactions can be ascribed to divalproex sodium alone, or the combination of divalproex sodium and other antiepilepsy drugs.
Table 3. Adverse Reactions Reported by ≥ 5% of Patients Treated with Divalproex Sodium During Placebo-Controlled Trial of Adjunctive Therapy for Complex Partial Seizures|
** Body System/Reaction** |
** Divalproex sodium** |
** Placebo** |
|
** Body as a Whole** | ||
|
Headache |
31 |
21 |
|
Asthenia |
27 |
7 |
|
Fever |
6 |
4 |
|
** Gastrointestinal System** | ||
|
Nausea |
48 |
14 |
|
Vomiting |
27 |
7 |
|
Abdominal Pain |
23 |
6 |
|
Diarrhea |
13 |
6 |
|
Anorexia |
12 |
0 |
|
Dyspepsia |
8 |
4 |
|
Constipation |
5 |
1 |
|
** Nervous System** | ||
|
Somnolence |
27 |
11 |
|
Tremor |
25 |
6 |
|
Dizziness |
25 |
13 |
|
Diplopia |
16 |
9 |
|
Amblyopia/Blurred Vision |
12 |
9 |
|
Ataxia |
8 |
1 |
|
Nystagmus |
8 |
1 |
|
Emotional Lability |
6 |
4 |
|
Thinking Abnormal |
6 |
0 |
|
Amnesia |
5 |
1 |
|
** Respiratory System** | ||
|
Flu Syndrome |
12 |
9 |
|
Infection |
12 |
6 |
|
Bronchitis |
5 |
1 |
|
Rhinitis |
5 |
4 |
|
** Other** | ||
|
Alopecia |
6 |
1 |
|
Weight Loss |
6 |
0 |
Table 4 lists treatment-emergent adverse reactions which were reported by ≥ 5% of patients in the high dose valproate group, and for which the incidence was greater than in the low dose group, in a controlled trial of divalproex sodium monotherapy treatment of complex partial seizures. Since patients were being titrated off another antiepilepsy drug during the first portion of the trial, it is not possible, in many cases, to determine whether the following adverse reactions can be ascribed to divalproex sodium alone, or the combination of valproate and other antiepilepsy drugs.
Table 4. Adverse Reactions Reported by ≥ 5% of Patients in the High Dose Group in the Controlled Trial of Valproate Monotherapy for Complex Partial Seizures1|
1Headache was the only adverse reaction that occurred in ≥ 5% of patients in the high dose group and at an equal or greater incidence in the low dose group. | ||
|
** Body System/Reaction** |
** High Dose** |
** Low Dose** |
|
** Body as a Whole** | ||
|
Asthenia |
21 |
10 |
|
** Digestive System** | ||
|
Nausea |
34 |
26 |
|
Diarrhea |
23 |
19 |
|
Vomiting |
23 |
15 |
|
Abdominal Pain |
12 |
9 |
|
Anorexia |
11 |
4 |
|
Dyspepsia |
11 |
10 |
|
** Hemic/Lymphatic System** | ||
|
Thrombocytopenia |
24 |
1 |
|
Ecchymosis |
5 |
4 |
|
** Metabolic/Nutritional** | ||
|
Weight Gain |
9 |
4 |
|
Peripheral Edema |
8 |
3 |
|
** Nervous System** | ||
|
Tremor |
57 |
19 |
|
Somnolence |
30 |
18 |
|
Dizziness |
18 |
13 |
|
Insomnia |
15 |
9 |
|
Nervousness |
11 |
7 |
|
Amnesia |
7 |
4 |
|
Nystagmus |
7 |
1 |
|
Depression |
5 |
4 |
|
** Respiratory System** | ||
|
Infection |
20 |
13 |
|
Pharyngitis |
8 |
2 |
|
Dyspnea |
5 |
1 |
|
** Skin and Appendages** | ||
|
Alopecia |
24 |
13 |
|
** Special Senses** | ||
|
Amblyopia/Blurred Vision |
8 |
4 |
|
Tinnitus |
7 |
1 |
The following additional adverse reactions were reported by greater than 1% but less than 5% of the 358 patients treated with valproate in the controlled trials of complex partial seizures:
Body as a Whole: Back pain, chest pain, malaise.
Cardiovascular System: Tachycardia, hypertension, palpitation.
Digestive System: Increased appetite, flatulence, hematemesis, eructation, pancreatitis, periodontal abscess.
Hemic and Lymphatic System: Petechia.
Metabolic and Nutritional Disorders: SGOT increased, SGPT increased.
Musculoskeletal System: Myalgia, twitching, arthralgia, leg cramps, myasthenia.
Nervous System: Anxiety, confusion, abnormal gait, paresthesia, hypertonia, incoordination, abnormal dreams, personality disorder.
Respiratory System: Sinusitis, cough increased, pneumonia, epistaxis.
Skin and Appendages: Rash, pruritus, dry skin.
Special Senses: Taste perversion, abnormal vision, deafness, otitis media.
Urogenital System: Urinary incontinence, vaginitis, dysmenorrhea, amenorrhea, urinary frequency.
6.3 Migraine
Based on two placebo-controlled clinical trials and their long term extension, valproate was generally well tolerated with most adverse reactions rated as mild to moderate in severity. Of the 202 patients exposed to valproate in the placebo-controlled trials, 17% discontinued for intolerance. This is compared to a rate of 5% for the 81 placebo patients. Including the long term extension study, the adverse reactions reported as the primary reason for discontinuation by ≥ 1% of 248 valproate-treated patients were alopecia (6%), nausea and/or vomiting (5%), weight gain (2%), tremor (2%), somnolence (1%), elevated SGOT and/or SGPT (1%), and depression (1%).
Table 5 includes those adverse reactions reported for patients in the placebo- controlled trials where the incidence rate in the divalproex sodium-treated group was greater than 5% and was greater than that for placebo patients.
Table 5. Adverse Reactions Reported by > 5% of Divalproex Sodium -Treated Patients During Migraine Placebo-Controlled Trials with a Greater Incidence Than Patients Taking Placebo1|
1The following adverse reactions occurred in at least 5% of divalproex sodium- treated patients and at an | ||
|
equal or greater incidence for placebo than for divalproex sodium** :** flu syndrome and pharyngitis. | ||
|
** Body System/Reaction** |
Divalproex sodium |
** Placebo** |
|
** Gastrointestinal System** | ||
|
Nausea |
31 |
10 |
|
Dyspepsia |
13 |
9 |
|
Diarrhea |
12 |
7 |
|
Vomiting |
11 |
1 |
|
Abdominal Pain |
9 |
4 |
|
Increased Appetite |
6 |
4 |
|
** Nervous System** | ||
|
Asthenia |
20 |
9 |
|
Somnolence |
17 |
5 |
|
Dizziness |
12 |
6 |
|
Tremor |
9 |
0 |
|
** Other** | ||
|
Weight Gain |
8 |
2 |
|
Back Pain |
8 |
6 |
|
Alopecia |
7 |
1 |
The following additional adverse reactions were reported by greater than 1% but not more than 5% of the 202 divalproex sodium-treated patients in the controlled clinical trials:
Body as a Whole: Chest pain, chills, face edema, fever and malaise.
Cardiovascular System: Vasodilatation.
Digestive System: Anorexia, constipation, dry mouth, flatulence, gastrointestinal disorder (unspecified), and stomatitis.
Hemic and Lymphatic System: Ecchymosis.
Metabolic and Nutritional Disorders: Peripheral edema, SGOT increase, and SGPT increase.
Musculoskeletal System: Leg cramps and myalgia.
Nervous System: Abnormal dreams, amnesia, confusion, depression, emotional lability, insomnia, nervousness, paresthesia, speech disorder, thinking abnormalities, and vertigo.
Respiratory System: Cough increased, dyspnea, rhinitis, and sinusitis.
Skin and Appendages: Pruritus and rash.
Special Senses: Conjunctivitis, ear disorder, taste perversion, and tinnitus.
Urogenital System: Cystitis, metrorrhagia, and vaginal hemorrhage.
6.4 Postmarketing Experience
The following adverse reactions have been identified during post approval use of divalproex sodium. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dermatologic: Hair texture changes, hair color changes, photosensitivity, erythema multiforme, toxic epidermal necrolysis, nail and nail bed disorders, and Stevens-Johnson syndrome.
Psychiatric: Emotional upset, psychosis, aggression, psychomotor hyperactivity, hostility, disturbance in attention, learning disorder, and behavioral deterioration.
Neurologic: Paradoxical convulsion, parkinsonism
There have been several reports of acute or subacute cognitive decline and behavioral changes (apathy or irritability) with cerebral pseudoatrophy on imaging associated with valproate therapy; both the cognitive/behavioral changes and cerebral pseudoatrophy reversed partially or fully after valproate discontinuation.
There have been reports of acute or subacute encephalopathy in the absence of elevated ammonia levels, elevated valproate levels, or neuroimaging changes. The encephalopathy reversed partially or fully after valproate discontinuation.
Musculoskeletal: Fractures, decreased bone mineral density, osteopenia, osteoporosis, and weakness.
Hematologic: Relative lymphocytosis, macrocytosis, leukopenia, acquired Pelger-Huet anomaly, anemia including macrocytic with or without folate deficiency, bone marrow suppression, pancytopenia, aplastic anemia, agranulocytosis, and acute intermittent porphyria.
Endocrine: Irregular menses, secondary amenorrhea, hyperandrogenism, hirsutism, elevated testosterone level, breast enlargement, galactorrhea, parotid gland swelling, polycystic ovary disease, decreased carnitine concentrations, hyponatremia, hyperglycinemia, and inappropriate ADH secretion.
There have been rare reports of Fanconi's syndrome occurring chiefly in children.
Metabolism and nutrition: Weight gain.
Reproductive: Aspermia, azoospermia, decreased sperm count, decreased spermatozoa motility, male infertility, and abnormal spermatozoa morphology.
Genitourinary: Enuresis, urinary tract infection, and tubulointerstitial nephritis.
Special Senses: Hearing loss.
Other: Allergic reaction, anaphylaxis, developmental delay, bone pain, bradycardia, and cutaneous vasculitis.
- Most common adverse reactions (reported >5%) are abdominal pain, accidental injury, alopecia, amblyopia/blurred vision, amnesia, anorexia, asthenia, ataxia, back pain, bronchitis, constipation, depression, diarrhea, diplopia, dizziness, dyspepsia, dyspnea, ecchymosis, emotional lability, fever, flu syndrome, headache, increased appetite, infection, insomnia, nausea, nervousness, nystagmus, peripheral edema, pharyngitis, rash, rhinitis, somnolence, thinking abnormal, thrombocytopenia, tinnitus, tremor, vomiting, weight gain, weight loss (6.1,6.2, 6.3).
- The safety and tolerability of valproate in pediatric patients were shown to be comparable to those in adults (8.4).
To report SUSPECTED ADVERSE REACTIONS, contact Unichem Pharmaceuticals (USA), Inc., at 1-866-562-4616 or FDA at 1-800-FDA-1088 or ww.fda.gov/medwatch
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Effects of Co-Administered Drugs on Valproate Clearance
Drugs that affect the level of expression of hepatic enzymes, particularly those that elevate levels of glucuronosyltransferases (such as ritonavir), may increase the clearance of valproate. For example, phenytoin, carbamazepine, and phenobarbital (or primidone) can double the clearance of valproate. Thus, patients on monotherapy will generally have longer half-lives and higher concentrations than patients receiving polytherapy with antiepilepsy drugs.
In contrast, drugs that are inhibitors of cytochrome P450 isozymes, e.g., antidepressants, may be expected to have little effect on valproate clearance because cytochrome P450 microsomal mediated oxidation is a relatively minor secondary metabolic pathway compared to glucuronidation and beta-oxidation.
Because of these changes in valproate clearance, monitoring of valproate and concomitant drug concentrations should be increased whenever enzyme inducing drugs are introduced or withdrawn.
The following list provides information about the potential for an influence of several commonly prescribed medications on valproate pharmacokinetics. The list is not exhaustive nor could it be, since new interactions are continuously being reported.
Drugs for which a potentially important interaction has been observed
Aspirin
A study involving the co-administration of aspirin at antipyretic doses (11 to 16 mg/kg) with valproate to pediatric patients (n=6) revealed a decrease in protein binding and an inhibition of metabolism of valproate. Valproate free fraction was increased 4-fold in the presence of aspirin compared to valproate alone. The β-oxidation pathway consisting of 2-E-valproic acid, 3-OH-valproic acid, and 3-keto valproic acid was decreased from 25% of total metabolites excreted on valproate alone to 8.3% in the presence of aspirin. Caution should be observed if valproate and aspirin are to be co-administered.
Carbapenem Antibiotics
A clinically significant reduction in serum valproic acid concentration has been reported in patients receiving carbapenem antibiotics (for example, ertapenem, imipenem, meropenem; this is not a complete list) and may result in loss of seizure control. The mechanism of this interaction is not well understood. Serum valproic acid concentrations should be monitored frequently after initiating carbapenem therapy. Alternative antibacterial or anticonvulsant therapy should be considered if serum valproic acid concentrations drop significantly or seizure control deteriorates [see Warnings and Precautions (5.13)].
Estrogen-Containing Hormonal Contraceptives
Estrogen-containing hormonal contraceptives may increase the clearance of valproate, which may result in decreased concentration of valproate and potentially increased seizure frequency. Prescribers should monitor serum valproate concentrations and clinical response when adding or discontinuing estrogen containing products.
Felbamate
A study involving the co-administration of 1,200 mg/day of felbamate with valproate to patients with epilepsy (n=10) revealed an increase in mean valproate peak concentration by 35% (from 86 to 115 mcg/mL) compared to valproate alone. Increasing the felbamate dose to 2,400 mg/day increased the mean valproate peak concentration to 133 mcg/mL (another 16% increase). A decrease in valproate dosage may be necessary when felbamate therapy is initiated.
Methotrexate
Methotrexate may decrease serum valproate levels and potentially result in increased frequency of seizures or bipolar symptoms. Prescribers should monitor serum valproate concentrations and clinical response when adding or discontinuing methotrexate and adjust valproate dosage, if necessary.
Rifampin
A study involving the administration of a single dose of valproate (7 mg/kg) 36 hours after 5 nights of daily dosing with rifampin (600 mg) revealed a 40% increase in the oral clearance of valproate. Valproate dosage adjustment may be necessary when it is co-administered with rifampin.
7.2 Effects of Valproate on Other Drugs
Valproate has been found to be a weak inhibitor of some P450 isozymes, epoxide hydrase, and glucuronosyltransferases.
The following list provides information about the potential for an influence of valproate co administration on the pharmacokinetics or pharmacodynamics of several commonly prescribed medications. The list is not exhaustive, since new interactions are continuously being reported.
Drugs for which a potentially important valproate interaction has been observed
Amitriptyline/Nortriptyline
Administration of a single oral 50 mg dose of amitriptyline to 15 normal volunteers (10 males and 5 females) who received valproate (500 mg BID) resulted in a 21% decrease in plasma clearance of amitriptyline and a 34% decrease in the net clearance of nortriptyline. Rare postmarketing reports of concurrent use of valproate and amitriptyline resulting in an increased amitriptyline level have been received. Concurrent use of valproate and amitriptyline has rarely been associated with toxicity. Monitoring of amitriptyline levels should be considered for patients taking valproate concomitantly with amitriptyline. Consideration should be given to lowering the dose of amitriptyline/nortriptyline in the presence of valproate.
Carbamazepine/carbamazepine-10,11-Epoxide
Serum levels of carbamazepine (CBZ) decreased 17% while that of carbamazepine-10,11- epoxide (CBZ-E) increased by 45% upon co-administration of valproate and CBZ to epileptic patients.
Clonazepam
The concomitant use of valproate and clonazepam may induce absence status in patients with a history of absence type seizures.
Diazepam
Valproate displaces diazepam from its plasma albumin binding sites and inhibits its metabolism. Co-administration of valproate (1,500 mg daily) increased the free fraction of diazepam (10 mg) by 90% in healthy volunteers (n=6). Plasma clearance and volume of distribution for free diazepam were reduced by 25% and 20%, respectively, in the presence of valproate. The elimination half-life of diazepam remained unchanged upon addition of valproate.
Ethosuximide
Valproate inhibits the metabolism of ethosuximide. Administration of a single ethosuximide dose of 500 mg with valproate (800 to 1,600 mg/day) to healthy volunteers (n=6) was accompanied by a 25% increase in elimination half-life of ethosuximide and a 15% decrease in its total clearance as compared to ethosuximide alone. Patients receiving valproate and ethosuximide, especially along with other anticonvulsants, should be monitored for alterations in serum concentrations of both drugs.
Lamotrigine
In a steady-state study involving 10 healthy volunteers, the elimination half- life of lamotrigine increased from 26 to 70 hours with valproate co- administration (a 165% increase). The dose of lamotrigine should be reduced when co-administered with valproate. Serious skin reactions (such as Stevens- Johnson syndrome and toxic epidermal necrolysis) have been reported with concomitant lamotrigine and valproate administration. See lamotrigine package insert for details on lamotrigine dosing with concomitant valproate administration.
Phenobarbital
Valproate was found to inhibit the metabolism of phenobarbital. Co- administration of valproate (250 mg BID for 14 days) with phenobarbital to normal subjects (n=6) resulted in a 50% increase in half-life and a 30% decrease in plasma clearance of phenobarbital (60 mg single-dose). The fraction of phenobarbital dose excreted unchanged increased by 50% in presence of valproate.
There is evidence for severe CNS depression, with or without significant elevations of barbiturate or valproate serum concentrations. All patients receiving concomitant barbiturate therapy should be closely monitored for neurological toxicity. Serum barbiturate concentrations should be obtained, if possible, and the barbiturate dosage decreased, if appropriate.
Primidone, which is metabolized to a barbiturate, may be involved in a similar interaction with valproate.
Phenytoin
Valproate displaces phenytoin from its plasma albumin binding sites and inhibits its hepatic metabolism. Co-administration of valproate (400 mg TID) with phenytoin (250 mg) in normal volunteers (n=7) was associated with a 60% increase in the free fraction of phenytoin. Total plasma clearance and apparent volume of distribution of phenytoin increased 30% in the presence of valproate. Both the clearance and apparent volume of distribution of free phenytoin were reduced by 25%.
In patients with epilepsy, there have been reports of breakthrough seizures occurring with the combination of valproate and phenytoin. The dosage of phenytoin should be adjusted as required by the clinical situation.
Propofol
The concomitant use of valproate and propofol may lead to increased blood levels of propofol. Reduce the dose of propofol when co-administering with valproate. Monitor patients closely for signs of increased sedation or cardiorespiratory depression.
Rufinamide
Based on a population pharmacokinetic analysis, rufinamide clearance was decreased by valproate. Rufinamide concentrations were increased by <16% to 70%, dependent on concentration of valproate (with the larger increases being seen in pediatric patients at high doses or concentrations of valproate). Patients stabilized on rufinamide before being prescribed valproate should begin valproate therapy at a low dose, and titrate to a clinically effective dose [see Dosage and Administration (2.5)]. Similarly, patients on valproate should begin at a rufinamide dose lower than 10 mg/kg per day (pediatric patients) or 400 mg per day (adults).
Tolbutamide
From in vitro experiments, the unbound fraction of tolbutamide was increased from 20% to 50% when added to plasma samples taken from patients treated with valproate. The clinical relevance of this displacement is unknown.
Warfarin
In an in vitro study, valproate increased the unbound fraction of warfarin by up to 32.6%. The therapeutic relevance of this is unknown; however, coagulation tests should be monitored if valproate therapy is instituted in patients taking anticoagulants.
Zidovudine
In six patients who were seropositive for HIV, the clearance of zidovudine (100 mg q8h) was decreased by 38% after administration of valproate (250 or 500 mg q8h); the half-life of zidovudine was unaffected.
7.3 Topiramate
Concomitant administration of valproate and topiramate has been associated with hyperammonemia with and without encephalopathy [see Contraindications (4)and Warnings and Precautions (5.6, 5.9, 5.10)]. Concomitant administration of topiramate with valproate has also been associated with hypothermia in patients who have tolerated either drug alone. It may be prudent to examine blood ammonia levels in patients in whom the onset of hypothermia has been reported [see Warnings and Precautions (5.9, 5.11)].
7.4 Cannabidiol
Concomitant administration of valproate and cannabidiol has been associated with an increased risk of ALT and/or AST elevation. This has been manageable by dose reduction or, in more severe cases, by discontinuation of one or both drugs. Liver function, including serum transaminase and total bilirubin levels, should be monitored during concomitant treatment [see Warnings and Precautions (5.1)].
- Hepatic enzyme-inducing drugs (e.g., phenytoin, carbamazepine, phenobarbital, primidone, rifampin) can increase valproate clearance, while enzyme inhibitors (e.g., felbamate) can decrease valproate clearance. Therefore increased monitoring of valproate and concomitant drug concentrations and dosage adjustment are indicated whenever enzyme-inducing or inhibiting drugs are introduced or withdrawn (7.1)
- Aspirin, carbapenem antibiotics, estrogen-containing hormonal contraceptives: Monitoring of valproate concentrations is recommended (7.1)
- Co-administration of valproate can affect the pharmacokinetics of other drugs (e.g. diazepam, ethosuximide, lamotrigine, phenytoin) by inhibiting their metabolism or protein binding displacement (7.2)
- Patients stabilized on rufinamide should begin valproate therapy at a low dose, and titrate to clinically effective dose (7.2)
- Dosage adjustment of amitriptyline/nortriptyline, propofol, warfarin, and zidovudine may be necessary if used concomitantly with divalproex sodium (7.2)
- Topiramate: Hyperammonemia and encephalopathy (5.10, 7.3)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Divalproex sodium delayed-release tablets, USP are supplied as:
125 mg orange colored tablets
250 mg pink colored tablets
500 mg reddish pink colored tablets
Tablets: 125 mg, 250 mg and 500 mg (3)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Divalproex sodium dissociates to the valproate ion in the gastrointestinal tract. The mechanisms by which valproate exerts its therapeutic effects have not been established. It has been suggested that its activity in epilepsy is related to increased brain concentrations of gamma-aminobutyric acid (GABA).
12.2 Pharmacodynamics
The relationship between plasma concentration and clinical response is not well documented. One contributing factor is the nonlinear, concentration dependent protein binding of valproate which affects the clearance of the drug. Thus, monitoring of total serum valproate cannot provide a reliable index of the bioactive valproate species.
For example, because the plasma protein binding of valproate is concentration dependent, the free fraction increases from approximately 10% at 40 mcg/mL to 18.5% at 130 mcg/mL. Higher than expected free fractions occur in the elderly, in hyperlipidemic patients, and in patients with hepatic and renal diseases.
Epilepsy
The therapeutic range in epilepsy is commonly considered to be 50 to 100 mcg/mL of total valproate, although some patients may be controlled with lower or higher plasma concentrations.
Mania
In placebo-controlled clinical trials of acute mania, patients were dosed to clinical response with trough plasma concentrations between 50 and 125 mcg/mL [see Dosage and Administration(2.1)].
12.3 Pharmacokinetics
Absorption/Bioavailability
Equivalent oral doses of divalproex sodium (divalproex sodium) products and valproic acid capsules deliver equivalent quantities of valproate ion systemically. Although the rate of valproate ion absorption may vary with the formulation administered (liquid, solid, or sprinkle), conditions of use (e.g., fasting or postprandial) and the method of administration (e.g., whether the contents of the capsule are sprinkled on food or the capsule is taken intact), these differences should be of minor clinical importance under the steady state conditions achieved in chronic use in the treatment of epilepsy.
However, it is possible that differences among the various valproate products in Tmax and Cmax could be important upon initiation of treatment. For example, in single dose studies, the effect of feeding had a greater influence on the rate of absorption of the tablet (increase in Tmax from 4 to 8 hours) than on the absorption of the sprinkle capsules (increase in Tmax from 3.3 to 4.8 hours).
While the absorption rate from the G.I. tract and fluctuation in valproate plasma concentrations vary with dosing regimen and formulation, the efficacy of valproate as an anticonvulsant in chronic use is unlikely to be affected. Experience employing dosing regimens from once-a-day to four-times-a-day, as well as studies in primate epilepsy models involving constant rate infusion, indicate that total daily systemic bioavailability (extent of absorption) is the primary determinant of seizure control and that differences in the ratios of plasma peak to trough concentrations between valproate formulations are inconsequential from a practical clinical standpoint. Whether or not rate of absorption influences the efficacy of valproate as an antimanic or antimigraine agent is unknown.
Co-administration of oral valproate products with food and substitution among the various divalproex sodium and valproic acid formulations should cause no clinical problems in the management of patients with epilepsy [see Dosage and Administration (2.2)]. Nonetheless, any changes in dosage administration, or the addition or discontinuance of concomitant drugs should ordinarily be accompanied by close monitoring of clinical status and valproate plasma concentrations.
Distribution
Protein Binding
The plasma protein binding of valproate is concentration dependent and the free fraction increases from approximately 10% at 40 mcg/mL to 18.5% at 130 mcg/mL. Protein binding of valproate is reduced in the elderly, in patients with chronic hepatic diseases, in patients with renal impairment, and in the presence of other drugs (e.g., aspirin). Conversely, valproate may displace certain protein-bound drugs (e.g., phenytoin, carbamazepine, warfarin, and tolbutamide) [see Drug Interactions (7.2)for more detailed information on the pharmacokinetic interactions of valproate with other drugs].
CNS Distribution
Valproate concentrations in cerebrospinal fluid (CSF) approximate unbound concentrations in plasma (about 10% of total concentration).
Metabolism
Valproate is metabolized almost entirely by the liver. In adult patients on monotherapy, 30-50% of an administered dose appears in urine as a glucuronide conjugate. Mitochondrial β-oxidation is the other major metabolic pathway, typically accounting for over 40% of the dose. Usually, less than 15-20% of the dose is eliminated by other oxidative mechanisms. Less than 3% of an administered dose is excreted unchanged in urine.
The relationship between dose and total valproate concentration is nonlinear; concentration does not increase proportionally with the dose, but rather, increases to a lesser extent due to saturable plasma protein binding. The kinetics of unbound drug are linear.
Elimination
Mean plasma clearance and volume of distribution for total valproate are 0.56 L/hr/1.73 m2 and 11 L/1.73 m2, respectively. Mean plasma clearance and volume of distribution for free valproate are 4.6 L/hr/1.73 m2 and 92 L/1.73 m2. Mean terminal half-life for valproate monotherapy ranged from 9 to 16 hours following oral dosing regimens of 250 to 1,000 mg.
The estimates cited apply primarily to patients who are not taking drugs that affect hepatic metabolizing enzyme systems. For example, patients taking enzyme-inducing antiepileptic drugs (carbamazepine, phenytoin, and phenobarbital) will clear valproate more rapidly. Because of these changes in valproate clearance, monitoring of antiepileptic concentrations should be intensified whenever concomitant antiepileptics are introduced or withdrawn.
Specific Populations
Effect of Age
Neonates
Children within the first two months of life have a markedly decreased ability to eliminate valproate compared to older children and adults. This is a result of reduced clearance (perhaps due to delay in development of glucuronosyltransferase and other enzyme systems involved in valproate elimination) as well as increased volume of distribution (in part due to decreased plasma protein binding). For example, in one study, the half-life in children under 10 days ranged from 10 to 67 hours compared to a range of 7 to 13 hours in children greater than 2 months.
Children
Pediatric patients (i.e., between 3 months and 10 years) have 50% higher clearances expressed on weight (i.e., mL/min/kg) than do adults. Over the age of 10 years, children have pharmacokinetic parameters that approximate those of adults.
Elderly
The capacity of elderly patients (age range: 68 to 89 years) to eliminate valproate has been shown to be reduced compared to younger adults (age range: 22 to 26 years). Intrinsic clearance is reduced by 39%; the free fraction is increased by 44%. Accordingly, the initial dosage should be reduced in the elderly [see Dosage and Administration (2.4)].
Effect of Sex
There are no differences in the body surface area adjusted unbound clearance between males and females (4.8±0.17 and 4.7±0.07 L/hr per 1.73 m2, respectively).
Effect of Race
The effects of race on the kinetics of valproate have not been studied.
Effect of Disease
Liver Disease
Liver disease impairs the capacity to eliminate valproate. In one study, the clearance of free valproate was decreased by 50% in 7 patients with cirrhosis and by 16% in 4 patients with acute hepatitis, compared with 6 healthy subjects. In that study, the half-life of valproate was increased from 12 to 18 hours. Liver disease is also associated with decreased albumin concentrations and larger unbound fractions (2 to 2.6 fold increase) of valproate. Accordingly, monitoring of total concentrations may be misleading since free concentrations may be substantially elevated in patients with hepatic disease whereas total concentrations may appear to be normal [see Boxed Warning, Contraindications (4), and Warnings and Precautions (5.1)].
Renal Disease
A slight reduction (27%) in the unbound clearance of valproate has been reported in patients with renal failure (creatinine clearance < 10 mL/minute); however, hemodialysis typically reduces valproate concentrations by about 20%. Therefore, no dosage adjustment appears to be necessary in patients with renal failure. Protein binding in these patients is substantially reduced; thus, monitoring total concentrations may be misleading.
Drug Interaction Studies with No Interaction or Likely Clinically Unimportant Interaction
Antacids
A study involving the co-administration of valproate 500 mg with commonly administered antacids (Maalox, Trisogel, and Titralac -160 mEq doses) did not reveal any effect on the extent of absorption of valproate.
Chlorpromazine
A study involving the administration of 100 to 300 mg/day of chlorpromazine to schizophrenic patients already receiving valproate (200 mg BID) revealed a 15% increase in trough plasma levels of valproate.
Haloperidol
A study involving the administration of 6 to 10 mg/day of haloperidol to schizophrenic patients already receiving valproate (200 mg BID) revealed no significant changes in valproate trough plasma levels.
Cimetidine and Ranitidine
Cimetidine and ranitidine do not affect the clearance of valproate.
Acetaminophen
Valproate had no effect on any of the pharmacokinetic parameters of acetaminophen when it was concurrently administered to three epileptic patients.
Clozapine
In psychotic patients (n=11), no interaction was observed when valproate was co-administered with clozapine.
Lithium
Co-administration of valproate (500 mg BID) and lithium carbonate (300 mg TID) to normal male volunteers (n=16) had no effect on the steady-state kinetics of lithium.
Lorazepam
Concomitant administration of valproate (500 mg BID) and lorazepam (1 mg BID) in normal male volunteers (n=9) was accompanied by a 17% decrease in the plasma clearance of lorazepam.
Olanzapine
No dose adjustment for olanzapine is necessary when olanzapine is administered concomitantly with valproate. Co-administration of valproate (500 mg BID) and olanzapine (5 mg) to healthy adults (n=10) caused 15% reduction in Cmax and 35% reduction in AUC of olanzapine.
Oral Contraceptive Steroids
Administration of a single-dose of ethinyloestradiol (50 mcg)/levonorgestrel (250 mcg) to 6 women on valproate (200 mg BID) therapy for 2 months did not reveal any pharmacokinetic interaction.
SPL MEDGUIDE SECTION
|
** MEDICATION GUIDE** | |||
|
** What is the most important information I should know about divalproex sodium delayed-release tablets?** ** Do not stop taking divalproex sodium delayed-release tablets without first talking to a healthcare provider.** Stopping divalproex sodium delayed- release tablets suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus). ** Divalproex sodium delayed-release tablets can cause serious side effects, including:** ** 1. Serious liver damage that can cause death, especially in children
younger than 2 years old and patients with mitochondrial disorders.** The
risk of getting this serious liver damage is more likely to happen within the
first 6 months of treatment.
In some cases, liver damage may continue even though the medicine is stopped. Your healthcare provider will do blood tests to check your liver before and during treatment with divalproex sodium delayed-release tablets. ** 2. Divalproex sodium delayed-release tablets may harm your unborn baby.**
** 3. Swelling (Inflammation) and bleeding (hemorrhaging) of your pancreas that can cause death.** ** Call your healthcare provider right away if you have any of these symptoms:**
** 4. Like other antiepileptic drugs, divalproex sodium delayed-release tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.** ** Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:**
** How can I watch for early symptoms of suicidal thoughts and actions?**
Call your healthcare provider between visits as needed, especially if you are worried about symptoms. Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. | |||
|
** What are divalproex sodium delayed-release tablets?** Divalproex sodium delayed-release tablets are prescription medicines used:
Divalproex delayed-release tablets are also used to prevent migraine
headaches. | |||
|
** Do not take divalproex sodium delayed-release tablets if you:**
| |||
|
** Before taking divalproex sodium delayed-release tablets, tell your healthcare provider about all of your medical conditions including if you:**
** Tell your healthcare provider about all the medicines you take,**
including prescription and over-the-counter medicines, vitamins, and herbal
supplements.
You can ask your healthcare provider or pharmacist for a list of these
medicines if you are not sure. | |||
|
** How should I take divalproex sodium delayed-release tablets?**
| |||
|
** What should I avoid while taking divalproex sodium delayed-release tablets?** *** Do not** drink alcohol while taking divalproex sodium delayed-release tablets. Divalproex sodium delayed-release tablets and alcohol can affect each other causing side effects such as sleepiness and dizziness.
| |||
|
** What are the possible side effects of divalproex sodium delayed-release tablets?** ** Call your healthcare provider right away if you have any of the symptoms listed below** . Your healthcare provider may do additional tests before and during your treatment with divalproex sodium delayed-release tablets. Your healthcare provider may reduce your dose, temporarily stop, or permanently stop treatment if you have certain side effects. | |||
|
** Divalproex sodium delayed-release tablets can cause serious side effects including:**
*** bleeding problems.** Call your healthcare provider if you have any symptoms of bleeding, including: | |||
|
| ||
|
| ||
|
| ||
|
*** increased ammonia levels in your blood.** High ammonia levels can seriously affect your mental activities, slow your alertness, make you feel tired, or cause vomiting (encephalopathy). This has happened when divalproex sodium delayed-release tablets are taken alone or with a medicine called topiramate. Call your health care provider if you have any of these symptoms. | |||
|
*** low body temperature (hypothermia).** A drop in your body temperature to less than 95°F can happen during treatment with divalproex sodium delayed-release tablets. Call your healthcare provider if you have any of the following symptoms: | |||
|
| ||
|
| ||
|
| ||
|
*** severe multiorgan reactions.** Treatment with divalproex sodium delayed-release tablets may cause severe multiorgan reactions that can be life-threatening or may lead to death. Stop taking divalproex sodium delayed-release tablets, and contact your healthcare provider or get medical help right away if you develop any of these symptoms of a severe skin reaction: | |||
|
| ||
|
| ||
|
| ||
|
| ||
|
*** drowsiness or sleepiness in the elderly.** This extreme drowsiness may cause you to eat or drink less than you normally would. Tell your healthcare provider if you are not able to eat or drink as you normally do. Your healthcare provider may start you at a lower dose of divalproex sodium delayed-release tablets. *** medicine residue in your stool.** Tell your healthcare provider if you have or think you may have medicine residue in your stool | |||
|
** The common side effects of divalproex sodium delayed-release tablets include:** | |||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
| |||
|
These are not all of the possible side effects of** divalproex sodium
delayed-release tablets** . | |||
|
** How should I store**** divalproex sodium delayed-release tablets?**
| |||
|
** Keep**** divalproex sodium delayed-release tablets and all medicines out of the reach of children.** | |||
|
** General information about the safe and effective use of divalproex sodium delayed-release tablets** Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use divalproex sodium delayed-release tablets for a condition for which it was not prescribed. Do not give divalproex sodium delayed-release tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about divalproex sodium delayed-release tablets that are written for health professionals. | |||
|
** What are the ingredients in divalproex sodium delayed-release tablets?** Active ingredient: divalproex sodium, USP Inactive ingredients: Microcrystalline cellulose, opadry II white 33G28707, povidone, pregelatinized starch (contains corn starch), silicon dioxide, simethicone, talc and vanillin. Opadry II white 33G28707 consists of hypromellose, lactose monohydrate, polyethylene glycol, titanium dioxide and triacetin. Imprinting ink contains ethanol, shellac glaze, iron oxide black, isopropyl alcohol, N-butyl alcohol and propylene glycol. Individual tablets also contain: ** 250 mg tablets:** Acryl EZE Pink which consists of D & C Red No. 30, FD & C Blue No. 2, iron oxide red, methacrylic acid copolymer, silica, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate. ** 500 mg tablets:** Acryl EZE Pink which consists of FD & C Red No. 40, methacrylic acid copolymer, silica, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate. ** Additional medication guides can be obtained by calling Unichem at 1-866-562-4616.** | |||
|
Manufactured by: Manufactured For: | |||
|
This Medication Guide has been approved by the U.S. Food and Drug
Administration. |
DESCRIPTION SECTION
11 DESCRIPTION
Divalproex sodium, USP is a stable co-ordination compound comprised of sodium valproate and valproic acid in a 1:1 molar relationship and formed during the partial neutralization of valproic acid with 0.5 equivalent of sodium hydroxide. Chemically it is designated as sodium hydrogen bis(2-propylpentanoate). Divalproex sodium, USP has the following structure:
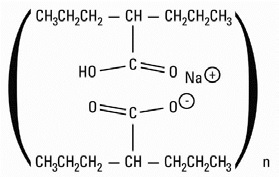
Divalproex sodium, USP occurs as a white powder with a characteristic odor.
Divalproex sodium delayed-release tablets, USP are for oral administration. Divalproex sodium delayed-release tablets, USP are supplied in three dosage strengths containing divalproex sodium, USP equivalent to 125 mg, 250 mg, or 500 mg of valproic acid.
Inactive Ingredients
Divalproex sodium delayed-release tablets, USP: Microcrystalline cellulose, opadry II white 33G28707, povidone, pregelatinized starch (contains corn starch), silicon dioxide, simethicone, talc and vanillin.
Opadry II white 33G28707 consists of hypromellose, lactose monohydrate, polyethylene glycol, titanium dioxide and triacetin.
Imprinting ink contains ethanol, shellac glaze, iron oxide black, isopropyl alcohol, N-butyl alcohol and propylene glycol.
In addition, individual tablets contain:
125 mg tablets: Acryl EZE Orange which consists of FD & C Yellow No. 6, methacrylic acid copolymer, silica, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate & yellow iron oxide.
250 mg tablets: Acryl EZE Pink which consists of D & C Red No. 30, FD & C Blue No. 2, iron oxide red, methacrylic acid copolymer, silica, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate.
500 mg tablets: Acryl EZE Pink which consists of FD & C Red No. 40, methacrylic acid copolymer, silica, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Mania
The effectiveness of divalproex sodium for the treatment of acute mania was demonstrated in two 3-week, placebo controlled, parallel group studies.
(1) Study 1: The first study enrolled adult patients who met DSM-III-R criteria for bipolar disorder and who were hospitalized for acute mania. In addition, they had a history of failing to respond to or not tolerating previous lithium carbonate treatment. Divalproex sodium was initiated at a dose of 250 mg tid and adjusted to achieve serum valproate concentrations in a range of 50-100 mcg/mL by day 7. Mean divalproex sodium doses for completers in this study were 1,118, 1,525, and 2,402 mg/day at Days 7, 14, and 21, respectively. Patients were assessed on the Young Mania Rating Scale (YMRS; score ranges from 0-60), an augmented Brief Psychiatric Rating Scale (BPRS-A), and the Global Assessment Scale (GAS). Baseline scores and change from baseline in the Week 3 endpoint (last-observation-carry-forward) analysis were as follows:
Table 6. Study 1|
1Mean score at baseline | |||
|
2Change from baseline to Week 3 (LOCF) | |||
|
3Difference in change from baseline to Week 3 endpoint (LOCF) between divalproex sodium and placebo | |||
|
** YMRS Total Score** | |||
|
** Group** |
** Baseline****1** |
** BL to Wk 3****2** |
** Difference****3** |
|
Placebo |
28.8 |
+ 0.2 | |
|
Divalproex sodium |
28.5 |
- 9.5 |
9.7 |
|
** BPRS-A Total Score** | |||
|
** Group** |
** Baseline****1** |
** BL to Wk 3****2** |
** Difference****3** |
|
Placebo |
76.2 |
+ 1.8 | |
|
Divalproex sodium |
76.4 |
-17.0 |
18.8 |
|
** GAS Score** | |||
|
** Group** |
** Baseline****1** |
** BL to Wk 3****2** |
** Difference****3** |
|
Placebo |
31.8 |
0.0 | |
|
Divalproex sodium |
30.3 |
+ 18.1 |
18.1 |
Divalproex sodium was statistically significantly superior to placebo on all three measures of outcome.
(2) Study 2: The second study enrolled adult patients who met Research Diagnostic Criteria for manic disorder and who were hospitalized for acute mania. Divalproex sodium was initiated at a dose of 250 mg tid and adjusted within a dose range of 750-2,500 mg/day to achieve serum valproate concentrations in a range of 40-150 mcg/mL. Mean divalproex sodium doses for completers in this study were 1,116, 1,683, and 2,006 mg/day at Days 7, 14, and 21, respectively. Study 2 also included a lithium group for which lithium doses for completers were 1,312, 1,869, and 1,984 mg/day at Days 7, 14, and 21, respectively. Patients were assessed on the Manic Rating Scale (MRS; score ranges from 11-63), and the primary outcome measures were the total MRS score, and scores for two subscales of the MRS, i.e., the Manic Syndrome Scale (MSS) and the Behavior and Ideation Scale (BIS). Baseline scores and change from baseline in the Week 3 endpoint (last-observation carry-forward) analysis were as follows:
Table 7. Study 2|
1Mean score at baseline | |||
|
2Change from baseline to Day 21 (LOCF) | |||
|
3Difference in change from baseline to Day 21 endpoint (LOCF) between divalproex sodium and placebo and lithium and placebo | |||
|
** MRS Total Score** | |||
|
** Group** |
** Baseline****1** |
** BL to Day 21****2** |
** Difference****3** |
|
Placebo |
38.9 |
- 4.4 | |
|
Lithium |
37.9 |
-10.5 |
6.1 |
|
Divalproex sodium |
38.1 |
- 9.5 |
5.1 |
|
** MSS Total Score** | |||
|
** Group** |
** Baseline****1** |
** BL to Day 21****2** |
** Difference****3** |
|
Placebo |
18.9 |
- 2.5 | |
|
Lithium |
18.5 |
- 6.2 |
3.7 |
|
Divalproex sodium |
18.9 |
- 6.0 |
3.5 |
|
** BIS Total Score** | |||
|
** Group** |
** Baseline****1** |
** BL to Day 21****2** |
** Difference****3** |
|
Placebo |
16.4 |
- 1.4 | |
|
Lithium |
16.0 |
- 3.8 |
2.4 |
|
Divalproex sodium |
15.7 |
- 3.2 |
1.8 |
Divalproex sodium was statistically significantly superior to placebo on all three measures of outcome. An exploratory analysis for age and gender effects on outcome did not suggest any differential responsiveness on the basis of age or gender.
A comparison of the percentage of patients showing ≥ 30% reduction in the symptom score from baseline in each treatment group, separated by study, is shown in Figure 1.
Figure 1
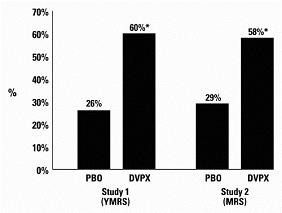
- p < 0.05
PBO = placebo, DVPX = Divalproex sodium
14.2 Epilepsy
The efficacy of valproate in reducing the incidence of complex partial seizures (CPS) that occur in isolation or in association with other seizure types was established in two controlled trials.
In one, multi-clinic, placebo controlled study employing an add-on design (adjunctive therapy), 144 patients who continued to suffer eight or more CPS per 8 weeks during an 8 week period of monotherapy with doses of either carbamazepine or phenytoin sufficient to assure plasma concentrations within the "therapeutic range" were randomized to receive, in addition to their original antiepilepsy drug (AED), either divalproex sodium or placebo. Randomized patients were to be followed for a total of 16 weeks. The following table presents the findings.
Table 8. Adjunctive Therapy Study Median Incidence of CPS per 8 Weeks
| |||
|
** Add-on** |
** Number** |
** Baseline** |
** Experimental** |
|
Divalproex sodium |
75 |
16.0 |
8.9* |
|
Placebo |
69 |
14.5 |
11.5 |
Figure 2 presents the proportion of patients (X axis) whose percentage reduction from baseline in complex partial seizure rates was at least as great as that indicated on the Y axis in the adjunctive therapy study. A positive percent reduction indicates an improvement (i.e., a decrease in seizure frequency), while a negative percent reduction indicates worsening. Thus, in a display of this type, the curve for an effective treatment is shifted to the left of the curve for placebo. This figure shows that the proportion of patients achieving any particular level of improvement was consistently higher for valproate than for placebo. For example, 45% of patients treated with valproate had a ≥ 50% reduction in complex partial seizure rate compared to 23% of patients treated with placebo.
Figure 2
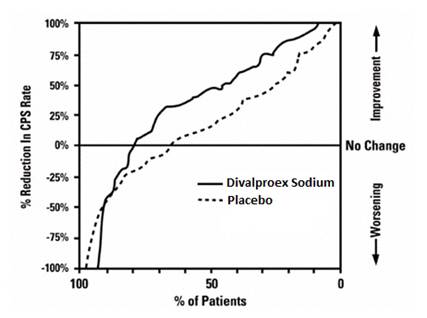
The second study assessed the capacity of valproate to reduce the incidence of CPS when administered as the sole AED. The study compared the incidence of CPS among patients randomized to either a high or low dose treatment arm. Patients qualified for entry into the randomized comparison phase of this study only if
- they continued to experience 2 or more CPS per 4 weeks during an 8 to 12 week long period of monotherapy with adequate doses of an AED (i.e., phenytoin, carbamazepine, phenobarbital, or primidone) and 2) they made a successful transition over a two week interval to valproate. Patients entering the randomized phase were then brought to their assigned target dose, gradually tapered off their concomitant AED and followed for an interval as long as 22 weeks. Less than 50% of the patients randomized, however, completed the study. In patients converted to divalproex sodium monotherapy, the mean total valproate concentrations during monotherapy were 71 and 123 mcg/mL in the low dose and high dose groups, respectively.
The following table presents the findings for all patients randomized who had at least one post randomization assessment.
Table 9. Monotherapy Study Median Incidence of CPS per 8 Weeks
| |||
|
** Treatment** |
** Number of Patients** |
** Baseline** |
** Randomized** |
|
High dose Divalproex sodium |
131 |
13.2 |
10.7* |
|
Low dose Divalproex sodium |
134 |
14.2 |
13.8 |
Figure 3 presents the proportion of patients (X axis) whose percentage reduction from baseline in complex partial seizure rates was at least as great as that indicated on the Y axis in the monotherapy study. A positive percent reduction indicates an improvement (i.e., a decrease in seizure frequency), while a negative percent reduction indicates worsening. Thus, in a display of this type, the curve for a more effective treatment is shifted to the left of the curve for a less effective treatment. This figure shows that the proportion of patients achieving any particular level of reduction was consistently higher for high dose valproate than for low dose valproate. For example, when switching from carbamazepine, phenytoin, phenobarbital or primidone monotherapy to high dose valproate monotherapy, 63% of patients experienced no change or a reduction in complex partial seizure rates compared to 54% of patients receiving low dose valproate.
Figure 3
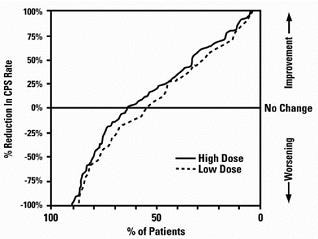
Information on pediatric studies is presented in section 8.
14.3 Migraine
The results of two multicenter, randomized, double-blind, placebo-controlled clinical trials established the effectiveness of divalproex sodium in the prophylactic treatment of migraine headache.
Both studies employed essentially identical designs and recruited patients with a history of migraine with or without aura (of at least 6 months in duration) who were experiencing at least 2 migraine headaches a month during the 3 months prior to enrollment. Patients with cluster headaches were excluded. Women of childbearing potential were excluded entirely from one study, but were permitted in the other if they were deemed to be practicing an effective method of contraception.
In each study following a 4-week single-blind placebo baseline period, patients were randomized, under double blind conditions, to divalproex sodium or placebo for a 12-week treatment phase, comprised of a 4-week dose titration period followed by an 8-week maintenance period. Treatment outcome was assessed on the basis of 4-week migraine headache rates during the treatment phase.
In the first study, a total of 107 patients (24 M, 83 F), ranging in age from 26 to 73 were randomized 2:1, divalproex sodium to placebo. Ninety patients completed the 8-week maintenance period. Drug dose titration, using 250 mg tablets, was individualized at the investigator's discretion. Adjustments were guided by actual/sham trough total serum valproate levels in order to maintain the study blind. In patients on divalproex sodium doses ranged from 500 to 2,500 mg a day. Doses over 500 mg were given in three divided doses (TID). The mean dose during the treatment phase was 1,087 mg/day resulting in a mean trough total valproate level of 72.5 mcg/mL, with a range of 31 to 133 mcg/mL.
The mean 4-week migraine headache rate during the treatment phase was 5.7 in the placebo group compared to 3.5 in the divalproex sodium group (see Figure 4). These rates were significantly different.
In the second study, a total of 176 patients (19 males and 157 females), ranging in age from 17 to 76 years, were randomized equally to one of three divalproex sodium dose groups (500, 1,000, or 1,500 mg/day) or placebo. The treatments were given in two divided doses (BID). One hundred thirty-seven patients completed the 8-week maintenance period. Efficacy was to be determined by a comparison of the 4-week migraine headache rate in the combined 1,000/1,500 mg/day group and placebo group.
The initial dose was 250 mg daily. The regimen was advanced by 250 mg every 4 days (8 days for 500 mg/day group), until the randomized dose was achieved. The mean trough total valproate levels during the treatment phase were 39.6, 62.5, and 72.5 mcg/mL in the divalproex sodium 500, 1,000, and 1,500 mg/day groups, respectively.
The mean 4-week migraine headache rates during the treatment phase, adjusted for differences in baseline rates, were 4.5 in the placebo group, compared to 3.3, 3.0, and 3.3 in the divalproex sodium 500, 1,000, and 1,500 mg/day groups, respectively, based on intent-to-treat results (see Figure 4). Migraine headache rates in the combined divalproex sodium 1,000/1,500 mg group were significantly lower than in the placebo group.
Figure 4 Mean 4-week Migraine Rates
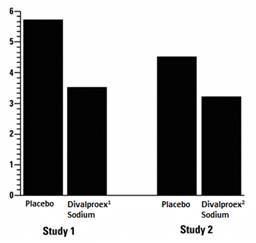
1 Mean dose of divalproex sodium was 1,087 mg/day.
2 Dose of divalproex sodium was 500 or 1,000 mg/day.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
250 mg: Pink colored, oval shaped, biconvex, enteric coated tablets, imprinted with "UL 250" on one side and plain on other side.
- NDC 71335-0008-1: 30 Tablets in a BOTTLE
- NDC 71335-0008-2: 60 Tablets in a BOTTLE
- NDC 71335-0008-3: 90 Tablets in a BOTTLE
- NDC 71335-0008-4: 120 Tablets in a BOTTLE
Store at 20° to 25°C (68° to 77°F) [see USP controlled Room Temperature].
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Divalproex sodium delayed-release tablets are intended for oral administration. Divalproex sodium delayed-release tablets should be swallowed whole and should not be crushed or chewed.
Patients should be informed to take divalproex sodium delayed-release tablets every day as prescribed. If a dose is missed it should be taken as soon as possible, unless it is almost time for the next dose. If a dose is skipped, the patient should not double the next dose.
2.1 Mania
Divalproex sodium delayed-release tablets are administered orally. The recommended initial dose is 750 mg daily in divided doses. The dose should be increased as rapidly as possible to achieve the lowest therapeutic dose which produces the desired clinical effect or the desired range of plasma concentrations. In placebo-controlled clinical trials of acute mania, patients were dosed to a clinical response with a trough plasma concentration between 50 and 125 mcg/mL. Maximum concentrations were generally achieved within 14 days. The maximum recommended dosage is 60 mg/kg/day.
There is no body of evidence available from controlled trials to guide a clinician in the longer term management of a patient who improves during divalproex sodium delayed-release tablets treatment of an acute manic episode. While it is generally agreed that pharmacological treatment beyond an acute response in mania is desirable, both for maintenance of the initial response and for prevention of new manic episodes, there are no data to support the benefits of divalproex sodium delayed-release tablets in such longer-term treatment. Although there are no efficacy data that specifically address longer-term antimanic treatment with divalproex sodium delayed-release tablets, the safety of divalproex sodium delayed-release tablets in long-term use is supported by data from record reviews involving approximately 360 patients treated with divalproex sodium delayed-release tablets for greater than 3 months.
2.2 Epilepsy
Divalproex sodium delayed-release tablets are administered orally. Divalproex sodium delayed-release tablets are indicated as monotherapy and adjunctive therapy in complex partial seizures in adults and pediatric patients down to the age of 10 years, and in simple and complex absence seizures. As the divalproex sodium delayed-release tablets dosage is titrated upward, concentrations of clonazepam, diazepam, ethosuximide, lamotrigine, tolbutamide, phenobarbital, carbamazepine, and/or phenytoin may be affected [see Drug Interactions (7.2)].
Complex Partial Seizures
For adults and children 10 years of age or older.
Monotherapy (Initial Therapy)
Divalproex sodium delayed-release tablets have not been systematically studied as initial therapy. Patients should initiate therapy at 10 to 15 mg/kg/day. The dosage should be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made.
The probability of thrombocytopenia increases significantly at total trough valproate plasma concentrations above 110 mcg/mL in females and 135 mcg/mL in males. The benefit of improved seizure control with higher doses should be weighed against the possibility of a greater incidence of adverse reactions.
Conversion to Monotherapy
Patients should initiate therapy at 10 to 15 mg/kg/day. The dosage should be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50-100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made. Concomitant antiepilepsy drug (AED) dosage can ordinarily be reduced by approximately 25% every 2 weeks. This reduction may be started at initiation of divalproex sodium delayed-release tablets therapy, or delayed by 1 to 2 weeks if there is a concern that seizures are likely to occur with a reduction. The speed and duration of withdrawal of the concomitant AED can be highly variable, and patients should be monitored closely during this period for increased seizure frequency.
Adjunctive Therapy
Divalproex sodium delayed-release tablets may be added to the patient's regimen at a dosage of 10 to 15 mg/kg/day. The dosage may be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made. If the total daily dose exceeds 250 mg, it should be given in divided doses.
In a study of adjunctive therapy for complex partial seizures in which patients were receiving either carbamazepine or phenytoin in addition to valproate, no adjustment of carbamazepine or phenytoin dosage was needed [see Clinical Studies (14.2)]. However, since valproate may interact with these or other concurrently administered AEDs as well as other drugs, periodic plasma concentration determinations of concomitant AEDs are recommended during the early course of therapy [see Drug Interactions (7)].
Simple and Complex Absence Seizures
The recommended initial dose is 15 mg/kg/day, increasing at one week intervals by 5 to 10 mg/kg/day until seizures are controlled or side effects preclude further increases. The maximum recommended dosage is 60 mg/kg/day. If the total daily dose exceeds 250 mg, it should be given in divided doses.
A good correlation has not been established between daily dose, serum concentrations, and therapeutic effect. However, therapeutic valproate serum concentrations for most patients with absence seizures is considered to range from 50 to 100 mcg/mL. Some patients may be controlled with lower or higher serum concentrations [see Clinical Pharmacology (12.3)].
As the divalproex sodium delayed-release tablets dosage is titrated upward, blood concentrations of phenobarbital and/or phenytoin may be affected [see Drug Interactions (7.2)].
Antiepilepsy drugs should not be abruptly discontinued in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life.
In epileptic patients previously receiving valproic acid therapy, divalproex sodium delayed-release tablets should be initiated at the same daily dose and dosing schedule. After the patient is stabilized on divalproex sodium delayed- release tablets, a dosing schedule of two or three times a day may be elected in selected patients.
2.3 Migraine
Divalproex sodium delayed-release tablets are indicated for prophylaxis of migraine headaches in adults.
Divalproex sodium delayed-release tablets are administered orally. The recommended starting dose is 250 mg twice daily. Some patients may benefit from doses up to 1,000 mg/day. In the clinical trials, there was no evidence that higher doses led to greater efficacy.
2.4 General Dosing Advice
Dosing in Elderly Patients
Due to a decrease in unbound clearance of valproate and possibly a greater sensitivity to somnolence in the elderly, the starting dose should be reduced in these patients. Dosage should be increased more slowly and with regular monitoring for fluid and nutritional intake, dehydration, somnolence, and other adverse reactions. Dose reductions or discontinuation of valproate should be considered in patients with decreased food or fluid intake and in patients with excessive somnolence. The ultimate therapeutic dose should be achieved on the basis of both tolerability and clinical response [see Warnings and Precautions (5.14), Use in Specific Populations (8.5)and Clinical Pharmacology (12.3)].
Dose-Related Adverse Reactions
The frequency of adverse effects (particularly elevated liver enzymes and thrombocytopenia) may be dose-related. The probability of thrombocytopenia appears to increase significantly at total valproate concentrations of ≥ 110 mcg/mL (females) or ≥ 135 mcg/mL (males) [see Warnings and Precautions (5.8)]. The benefit of improved therapeutic effect with higher doses should be weighed against the possibility of a greater incidence of adverse reactions.
G.I. Irritation
Patients who experience G.I. irritation may benefit from administration of the drug with food or by slowly building up the dose from an initial low level.
2.5 Dosing in Patients Taking Rufinamide
Patients stabilized on rufinamide before being prescribed valproate should begin valproate therapy at a low dose, and titrate to a clinically effective dose [see Drug Interactions (7.2)].
- Divalproex sodium delayed-release tablet is administered orally in divided doses. Divalproex sodium delayed-release tablets should be swallowed whole and should not be crushed or chewed (2.1, 2.2).
- Mania: Initial dose is 750 mg daily, increasing as rapidly as possible to achieve therapeutic response or desired plasma level (2.1). The maximum recommended dosage is 60 mg/kg/day (2.1, 2.2).
- Complex Partial Seizures: Start at 10 to 15 mg/kg/day, increasing at 1 week intervals by 5 to 10 mg/kg/day to achieve optimal clinical response; if response is not satisfactory, check valproate plasma level; see full prescribing information for conversion to monotherapy (2.2). The maximum recommended dosage is 60 mg/kg/day (2.1, 2.2).
- Absence Seizures: Start at 15 mg/kg/day, increasing at 1 week intervals by 5 to 10 mg/kg/day until seizure control or limiting side effects (2.2). The maximum recommended dosage is 60 mg/kg/day (2.1, 2.2).
- Migraine: The recommended starting dose is 250 mg twice daily, thereafter increasing to a maximum of 1,000 mg/day as needed (2.3).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Hepatotoxicity
Warn patients and guardians that nausea, vomiting, abdominal pain, anorexia, diarrhea, asthenia, and/or jaundice can be symptoms of hepatotoxicity and, therefore, require further medical evaluation promptly [see Warnings and Precautions (5.1)].
Pancreatitis
Warn patients and guardians that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis and, therefore, require further medical evaluation promptly [seeWarnings and Precautions (5.5)].
Birth Defects and Decreased IQ
Inform pregnant women and women of childbearing potential (including girls beginning the onset of puberty) that use of valproate during pregnancy increases the risk of birth defects, decreased IQ, and neurodevelopmental disorders in children who were exposed in utero. Advise women to use effective contraception while taking valproate. When appropriate, counsel these patients about alternative therapeutic options. This is particularly important when valproate use is considered for a condition not usually associated with permanent injury or death such as prophylaxis of migraine headache [see Contraindications (4)]. Advise patients to read the Medication Guide, which appears as the last section of the labeling [seeWarnings and Precautions (5.2, 5.3, 5.4) and Use in Specific Populations (8.1)].
Pregnancy Registry
Advise women of childbearing potential to discuss pregnancy planning with their doctor and to contact their doctor immediately if they think they are pregnant.
Encourage women who are taking divalproex sodium to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 or visit the website http://www.aedpregnancyregistry.org/ [see Use in Specific Populations(8.1)].
Suicidal Thinking and Behavior
Counsel patients, their caregivers, and families that AEDs, including divalproex sodium delayed-release tablets, may increase the risk of suicidal thoughts and behavior and to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients, caregivers, and families to report behaviors of concern immediately to the healthcare providers [see Warnings andPrecautions (5.7)].
Hyperammonemia
Inform patients of the signs and symptoms associated with hyperammonemic encephalopathy and to notify the prescriber if any of these symptoms occur [see Warnings and Precautions (5.9,5.10)].
CNS Depression
Since valproate products may produce CNS depression, especially when combined with another CNS depressant (e.g., alcohol), advise patients not to engage in hazardous activities, such as driving an automobile or operating dangerous machinery, until it is known that they do not become drowsy from the drug.
Multiorgan Hypersensitivity Reactions
Instruct patients that a fever associated with other organ system involvement (rash, lymphadenopathy, etc.) may be drug-related and should be reported to the physician immediately [see Warnings and Precautions (5.12)].
Medication Residue in the Stool
Instruct patients to notify their healthcare provider if they notice a medication residue in the stool [see Warnings and Precautions (5.18)].
18-R-04/2024

