Itch Relief
Carring Mill Itch Relief Pump Spray
3cd1933c-6341-50d6-e063-6294a90aca32
HUMAN OTC DRUG LABEL
Aug 19, 2025
FSA Store Inc.
DUNS: 049283340
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Diphenhydramine HCl and Zinc Acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
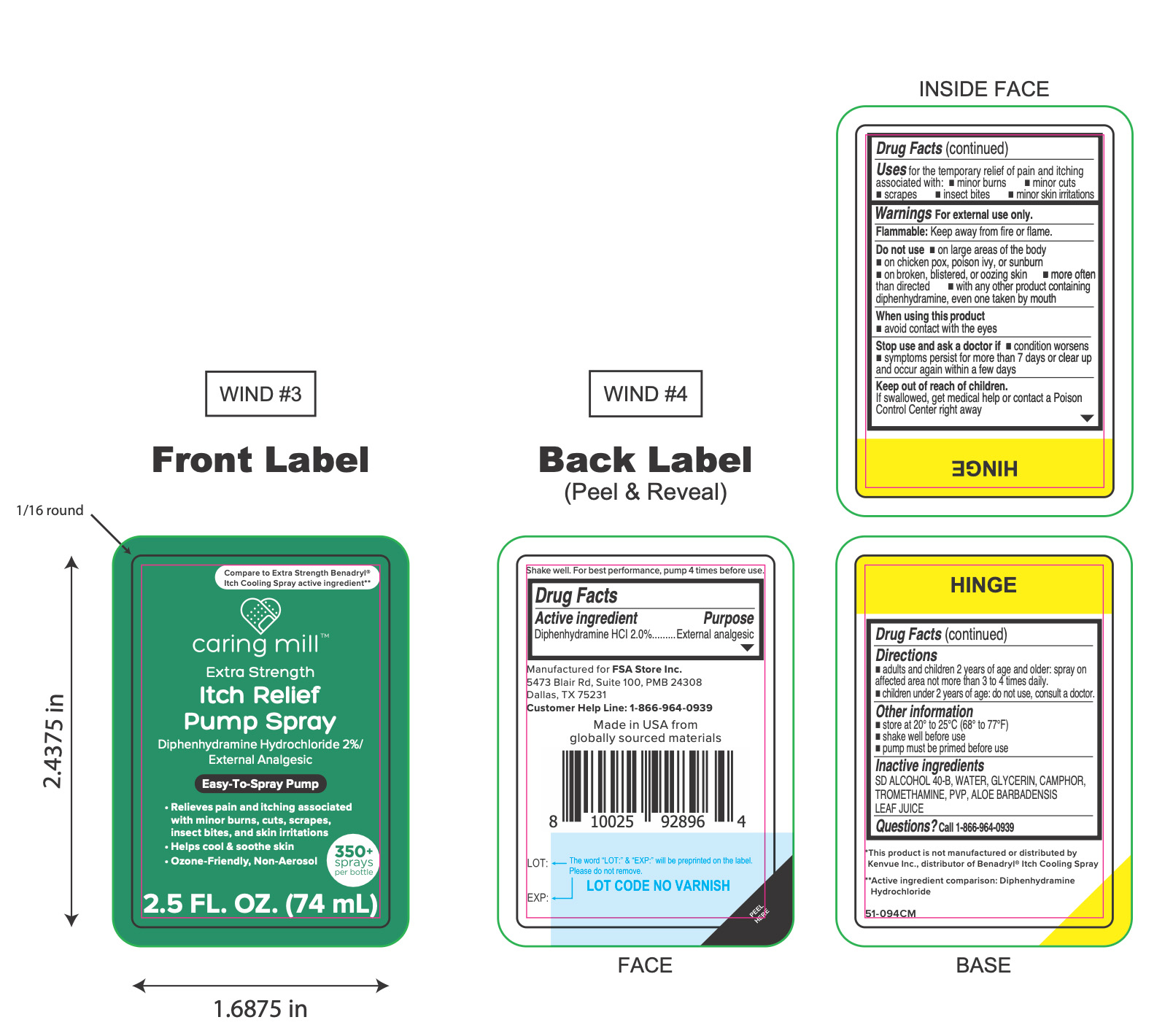
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
caring mill
Extra Strength
Itch Relief
Pump Spray
Diphenhydramine Hydrochloride 2%/
External Analgesic
Easy-To-Spray Pump
- Relieves pain and itching associated
with minor burns, cuts, scrapes,
insect bites, and skin irritations
- Helps cool & soothe skin
- Ozone-Friendly, Non-Aerosol
2.5 FL. OZ. (74 mL)
INDICATIONS & USAGE SECTION
Uses
for the temporary relief of pain and itching associated with:
- minor burns
- minor cuts
- scrapes
- insect bites
- minor skin irritations
OTC - ACTIVE INGREDIENT SECTION
Active ingredients
Diphenhydramine HCl 2.0%,
OTC - PURPOSE SECTION
Purpose
External analgesic
WARNINGS SECTION
Warnings
For external use only.
Flammable:
Keep away from fire or flame.
Do not use
- on large areas of the body
- on chicken pox, poison ivy, or sunburn
- on broken, blistered, or oozing skin
- more often than directed
- with any other product containing diphenhydramine, even one taken by mouth
When using this product
- avoid contact with the eyes
Stop use and ask doctor if
- conditions worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- adults and children 2 years of age and older: spray on affected area not more than 3 to 4 times daily.
- children under 2 years of age: do not use, consult a doctor.
OTHER SAFETY INFORMATION
Other information
- store between 20° to 25°C (68° to 77°F)
- shake well before use
- pump must be primed before use
INACTIVE INGREDIENT SECTION
Inactive ingredients
SD ALCOHOL 40-B, WATER, GLYCERIN, CAMPHOR, TROMETHAMINE, PVP, ALOE BARBADENSIS LEAF JUICE
OTC - QUESTIONS SECTION
Questions?
1-866-964-0939
