Carvedilol
These highlights do not include all the information needed to use CARVEDILOL TABLETS safely and effectively. See full prescribing information for CARVEDILOL TABLETS. CARVEDILOL tablets for oral use Initial U.S. Approval: 1995INDICATIONS AND USAGE Carvedilol tablets are an alpha-/beta-adrenergic blocking agent indicated for the treatment of: • mild to severe chronic heart failure (1.1) • left ventricular dysfunction following myocardial infarction in clinically stable patients (1.2) • hypertension (1.3) DOSAGE AND ADMINISTRATION Take with food. Individualize dosage and monitor during up-titration. (2) • Heart failure: Start at 3.125 mg twice daily and increase to 6.25, 12.5, and then 25 mg twice daily over intervals of at least 2 weeks. Maintain lower doses if higher doses are not tolerated. (2.1) • Left ventricular dysfunction following myocardial infarction: Start at 6.25 mg twice daily and increase to 12.5 mg then 25 mg twice daily after intervals of 3 to 10 days. A lower starting dose or slower titration may be used. (2.2) • Hypertension: Start at 6.25 mg twice daily and increase if needed for blood pressure control to 12.5 mg then 25 mg twice daily over intervals of 1 to 2 weeks. (2.3) DOSAGE FORMS AND STRENGTHS Tablets: 3.125 mg, 6.25 mg, 12.5 mg, 25 mg (3) CONTRAINDICATIONS • Bronchial asthma or related bronchospastic conditions. (4) • Second- or third-degree AV block. (4) • Sick sinus syndrome. (4) • Severe bradycardia (unless permanent pacemaker in place). (4) • Patients in cardiogenic shock or decompensated heart failure requiring the use of IV inotropic therapy. (4) • Severe hepatic impairment. (2.4, 4) • History of serious hypersensitivity reaction (e.g., Stevens-Johnson syndrome, anaphylactic reaction, angioedema) to any component of this medication or other medications containing carvedilol. (4) WARNINGS AND PRECAUTIONS • Acute exacerbation of coronary artery disease upon cessation of therapy: Do not abruptly discontinue. (5.1) • Bradycardia, hypotension, worsening heart failure/fluid retention may occur. Reduce the dose as needed. (5.2, 5.3, 5.4) • Non-allergic bronchospasm (e.g., chronic bronchitis and emphysema): Avoid β-blockers. (4) However, if deemed necessary, use with caution and at lowest effective dose. (5.5) • Diabetes: Monitor glucose as β-blockers may mask symptoms of hypoglycemia or worsen hyperglycemia. (5.6) ADVERSE REACTIONS Most common adverse events (6.1): • Heart failure and left ventricular dysfunction following myocardial infarction (≥ 10%): Dizziness, fatigue, hypotension, diarrhea, hyperglycemia, asthenia, bradycardia, weight increase • Hypertension (≥ 5%): Dizziness To report SUSPECTED ADVERSE REACTIONS, contact Glenmark Pharmaceuticals Inc., USA at 1 (888) 721-7115 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS • CYP P450 2D6 enzyme inhibitors may increase and rifampin may decrease carvedilol levels. (7.1, 7.5) • Hypotensive agents (e.g., reserpine, MAO inhibitors, clonidine) may increase the risk of hypotension and/or severe bradycardia. (7.2) • Cyclosporine or digoxin levels may increase. (7.3, 7.4) • Both digitalis glycosides and β-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia. (7.4) • Amiodarone may increase carvedilol levels resulting in further slowing of the heart rate or cardiac conduction. (7.6) • Verapamil- or diltiazem-type calcium channel blockers may affect ECG and/or blood pressure. (7.7) • Insulin and oral hypoglycemics action may be enhanced. (7.8) See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 9/2019
9236e7cc-2905-c463-e053-2a95a90a30a1
HUMAN PRESCRIPTION DRUG LABEL
Dec 16, 2020
St. Mary's Medical Park Pharmacy
DUNS: 063050751
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Carvedilol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

PATIENT COUNSELING INFORMATION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Patients taking carvedilol should be advised of the following:
•
Patients should take carvedilol with food.
•
Patients should not interrupt or discontinue using carvedilol without a
physician’s advice.
•
Patients with heart failure should consult their physician if they experience
signs or symptoms of worsening heart failure such as weight gain or increasing
shortness of breath.
•
Patients may experience a drop in blood pressure when standing, resulting in
dizziness and, rarely, fainting. Patients should sit or lie down when these
symptoms of lowered blood pressure occur.
•
If experiencing dizziness or fatigue, patients should avoid driving or
hazardous tasks.
•
Patients should consult a physician if they experience dizziness or faintness,
in case the dosage should be adjusted.
•
Diabetic patients should report any changes in blood sugar levels to their
physician.
•
Contact lens wearers may experience decreased lacrimation.
Manufactured by:
Glenmark Pharmaceuticals Ltd.
India
Manufactured for:
Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
September 2019
Patient Package Insert
Carvedilol (kar´ve dil ol) Tablets, USP
Read the Patient Information that comes with carvedilol tablets before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about carvedilol tablets, ask your doctor or pharmacist.
What are Carvedilol Tablets?
Carvedilol tablets are a prescription medicine that belongs to a group of medicines called “beta-blockers”.
Carvedilol tablets are used, often with other medicines, for the following conditions:
•
to treat patients with certain types of heart failure
•
to treat patients who had a heart attack that worsened how well the heart
pumps
•
to treat patients with high blood pressure (hypertension)
Carvedilol tablets are not approved for use in children under 18 years of age.
Who should not take Carvedilol Tablets?
Do not take carvedilol tablets if you:
•
have severe heart failure and are hospitalized in the intensive care unit or
require certain intravenous medications that help support circulation
(inotropic medications).
•
are prone to asthma or other breathing problems.
•
have a slow heartbeat or a heart that skips a beat (irregular heartbeat).
•
have liver problems.
•
are allergic to any of the ingredients in carvedilol tablets. The active
ingredient is carvedilol. See the end of this leaflet for a list of all the
ingredients in carvedilol tablets.
What should I tell my doctor before taking Carvedilol Tablets?
Tell your doctor about all of your medical conditions, including if you:
•
have asthma or other lung problems (such as bronchitis or emphysema).
•
have problems with blood flow in your feet and legs (peripheral vascular
disease). Carvedilol tablets can make some of your symptoms worse.
•
have diabetes.
•
have thyroid problems.
•
have a condition called pheochromocytoma.
•
have had severe allergic reactions.
•
are pregnant or trying to become pregnant. It is not known if carvedilol is
safe for your unborn baby. You and your doctor should talk about the best way
to control your high blood pressure during pregnancy.
•
are breastfeeding. It is not known if carvedilol passes into your breast milk.
Talk with your doctor about the best way to feed your baby if you are taking
carvedilol tablets.
•
are scheduled for surgery and will be given anesthetic agents.
•
are scheduled for cataract surgery and have taken or are currently taking
carvedilol tablets.
•
are taking prescription or over-the-counter medicines, vitamins, and herbal
supplements. Carvedilol and certain other medicines can affect each other and
cause serious side effects. Carvedilol may affect the way other medicines
work. Also, other medicines may affect how well carvedilol works.
Keep a list of all the medicines you take. Show this list to your doctor and pharmacist before you start a new medicine.
How should I take Carvedilol Tablets?
It is important for you to take your medicine every day as directed by your doctor. If you stop taking carvedilol tablets suddenly, you could have chest pain and/or a heart attack. If your doctor decides that you should stop taking carvedilol tablets, your doctor may slowly lower your dose over a period of time before stopping it completely.
•
Take carvedilol tablets exactly as prescribed. Your doctor will tell you how
many tablets to take and how often. In order to minimize possible side
effects, your doctor might begin with a low dose and then slowly increase the
dose.
•
Do not stop taking carvedilol tablets and do not change the amount of
carvedilol tablets you take without talking to your doctor.
•
Tell your doctor if you gain weight or have trouble breathing while taking
carvedilol tablets.
•
Take carvedilol tablets with food.
•
If you miss a dose of carvedilol tablets, take your dose as soon as you
remember, unless it is time to take your next dose. Take your next dose at the
usual time. Do not take 2 doses at the same time.
•
If you take too many carvedilol tablets, call your doctor or poison control
center right away.
What should I avoid while taking Carvedilol Tablets?
•
Carvedilol tablets can cause you to feel dizzy, tired, or faint. Do not drive
a car, use machinery, or do anything that needs you to be alert if you have
these symptoms.
What are possible side effects of Carvedilol Tablets?
•
Low blood pressure (which may cause dizziness or fainting when you stand up).
If these happen, sit or lie down right away and tell your doctor.
•
Tiredness. If you feel tired or dizzy you should not drive, use machinery, or
do anything that needs you to be alert.
•
Slow heartbeat.
•
Changes in your blood sugar. If you have diabetes, tell your doctor if you
have any changes in your blood sugar levels.
•
Carvedilol tablets may hide some of the symptoms of low blood sugar,
especially a fast heartbeat.
•
Carvedilol tablets may mask the symptoms of hyperthyroidism (overactive
thyroid).
•
Worsening of severe allergic reactions.
•
Rare but serious allergic reactions (including hives or swelling of the face,
lips, tongue, and/or throat that may cause difficulty in breathing or
swallowing) have happened in patients who were on carvedilol tablets. These
reactions can be life-threatening.
Other side effects of carvedilol tablets include shortness of breath, weight gain, diarrhea, and fewer tears or dry eyes that become bothersome if you wear contact lenses.
Call your doctor if you have any side effects that bother you or don’t go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Carvedilol Tablets?
•
Store carvedilol tablets at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature]. Protect from moisture. Dispense in a tight container.
•
Safely, throw away carvedilol tablets that are out of date or no longer
needed.
•
Keep carvedilol tablets and all medicines out of the reach of children.
General Information about Carvedilol Tablets
Medicines are sometimes prescribed for conditions other than those described in patient information leaflets. Do not use carvedilol tablets for a condition for which it was not prescribed. Do not give carvedilol tablets to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about carvedilol tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about carvedilol tablets that is written for healthcare professionals. You can also find out more about carvedilol tablets by calling 1 (888) 721-7115. This call is free.
What are the ingredients in Carvedilol Tablets?
Active Ingredient: carvedilol.
Inactive Ingredients: colloidal silicon dioxide, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, polysorbate 80, povidone and titanium dioxide.
Carvedilol tablets, USP come in the following strengths: 3.125 mg, 6.25 mg, 12.5 mg, 25 mg.
What is high blood pressure (hypertension)?
Blood pressure is the force of blood in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. High blood pressure makes the heart work harder to pump blood through the body and causes damage to blood vessels. Carvedilol tablets can help your blood vessels relax so your blood pressure is lower. Medicines that lower blood pressure may lower your chance of having a stroke or heart attack.
Manufactured by:
Glenmark Pharmaceuticals Ltd.
India
Manufactured for:
Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
September 2019
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Heart Failure
A total of 6,975 subjects with mild to severe heart failure were evaluated in placebo-controlled trials of carvedilol.
Mild-to-Moderate Heart Failure:
Carvedilol was studied in 5 multicenter, placebo-controlled trials, and in 1 active-controlled trial (COMET trial) involving subjects with mild-to-moderate heart failure.
Four U.S. multicenter, double-blind, placebo-controlled trials enrolled 1,094 subjects (696 randomized to carvedilol) with NYHA class II-III heart failure and ejection fraction less than or equal to 0.35. The vast majority were on digitalis, diuretics, and an ACE inhibitor at trial entry. Patients were assigned to the trials based upon exercise ability. An Australia-New Zealand double-blind, placebo-controlled trial enrolled 415 subjects (half randomized to carvedilol) with less severe heart failure. All protocols excluded subjects expected to undergo cardiac transplantation during the 7.5 to 15 months of double-blind follow-up. All randomized subjects had tolerated a 2-week course on carvedilol 6.25 mg twice daily.
In each trial, there was a primary end point, either progression of heart failure (1 U.S. trial) or exercise tolerance (2 U.S. trials meeting enrollment goals and the Australia-New Zealand trial). There were many secondary end points specified in these trials, including NYHA classification, patient and physician global assessments, and cardiovascular hospitalization. Other analyses not prospectively planned included the sum of deaths and total cardiovascular hospitalizations. In situations where the primary end points of a trial do not show a significant benefit of treatment, assignment of significance values to the other results is complex, and such values need to be interpreted cautiously.
The results of the U.S. and Australia-New Zealand trials were as follows:
**Slowing Progression of Heart Failure:**One U.S. multicenter trial (366 subjects) had as its primary end point the sum of cardiovascular mortality, cardiovascular hospitalization, and sustained increase in heart failure medications. Heart failure progression was reduced, during an average follow- up of 7 months, by 48% (P = 0.008).
In the Australia-New Zealand trial, death and total hospitalizations were reduced by about 25% over 18 to 24 months. In the 3 largest U.S. trials, death and total hospitalizations were reduced by 19%, 39%, and 49%, nominally statistically significant in the last 2 trials. The Australia-New Zealand results were statistically borderline.
**Functional Measures:**None of the multicenter trials had NYHA classification as a primary end point, but all such trials had it as a secondary end point. There was at least a trend toward improvement in NYHA class in all trials. Exercise tolerance was the primary end point in 3 trials; in none was a statistically significant effect found.
Subjective Measures: Health-related quality of life, as measured with a standard questionnaire (a primary end point in 1 trial), was unaffected by carvedilol. However, patients’ and investigators’ global assessments showed significant improvement in most trials.
**Mortality:**Death was not a pre-specified end point in any trial, but was analyzed in all trials. Overall, in these 4 U.S. trials, mortality was reduced, nominally significantly so in 2 trials.
The COMET Trial:
In this double-blind trial, 3,029 subjects with NYHA class II-IV heart failure (left ventricular ejection fraction less than or equal to 35%) were randomized to receive either carvedilol (target dose: 25 mg twice daily) or immediate- release metoprolol tartrate (target dose: 50 mg twice daily). The mean age of the subjects was approximately 62 years, 80% were males, and the mean left ventricular ejection fraction at baseline was 26%. Approximately 96% of the subjects had NYHA class II or III heart failure. Concomitant treatment included diuretics (99%), ACE inhibitors (91%), digitalis (59%), aldosterone antagonists (11%), and “statin” lipid-lowering agents (21%). The mean duration of follow-up was 4.8 years. The mean dose of carvedilol was 42 mg per day.
The trial had 2 primary end points: all-cause mortality and the composite of death plus hospitalization for any reason. The results of COMET are presented in Table 3 below. All-cause mortality carried most of the statistical weight and was the primary determinant of the trial size. All-cause mortality was 34% in the subjects treated with carvedilol and was 40% in the immediate-release metoprolol group (P = 0.0017; hazard ratio = 0.83, 95%CI 0.74 to 0.93). The effect on mortality was primarily due to a reduction in cardiovascular death. The difference between the 2 groups with respect to the composite end point was not significant (P = 0.122). The estimated mean survival was 8 years with carvedilol and 6.6 years with immediate-release metoprolol.
Table 3. Results of COMET
|
End Point |
Carvedilol N=1,511 |
Metoprolol N=1,518 |
Hazard Ratio |
(95% CI) |
|
All-cause mortality |
34% |
40% |
0.83 |
0.74-0.93 |
|
Mortality + all hospitalization |
74% |
76% |
0.94 |
0.86-1.02 |
|
Cardiovascular death |
30% |
35% |
0.80 |
0.70-0.90 |
|
14% 11% 0.9% |
17% 13% 2.5% |
0.81 0.83 0.33 |
0.68-0.97 0.67-1.02 0.18-0.62 |
It is not known whether this formulation of metoprolol at any dose or this low dose of metoprolol in any formulation has any effect on survival or hospitalization in patients with heart failure. Thus, this trial extends the time over which carvedilol manifests benefits on survival in heart failure, but it is not evidence that carvedilol improves outcome over the formulation of metoprolol (TOPROL-XL ®) with benefits in heart failure.
Severe Heart Failure (COPERNICUS):
In a double-blind trial (COPERNICUS), 2,289 subjects with heart failure at rest or with minimal exertion and left ventricular ejection fraction less than 25% (mean 20%), despite digitalis (66%), diuretics (99%), and ACE inhibitors (89%), were randomized to placebo or carvedilol. Carvedilol was titrated from a starting dose of 3.125 mg twice daily to the maximum tolerated dose or up to 25 mg twice daily over a minimum of 6 weeks. Most subjects achieved the target dose of 25 mg. The trial was conducted in Eastern and Western Europe, the United States, Israel, and Canada. Similar numbers of subjects per group (about 100) withdrew during the titration period.
The primary end point of the trial was all-cause mortality, but cause-specific mortality and the risk of death or hospitalization (total, cardiovascular [CV], or heart failure [HF]) were also examined. The developing trial data were followed by a data monitoring committee, and mortality analyses were adjusted for these multiple looks. The trial was stopped after a median follow-up of 10 months because of an observed 35% reduction in mortality (from 19.7% per patient year on placebo to 12.8% on carvedilol, hazard ratio 0.65, 95% CI 0.52 to 0.81, P = 0.0014, adjusted) (see Figure 1). The results of COPERNICUS are shown in Table 4.
Table 4. Results of COPERNICUS Trial in Subjects with Severe Heart Failure
|
End Point |
Placebo (n=1,133) |
Carvedilol (n=1,156) |
Hazard Ratio (95% CI) |
% Reduction |
NominalPvalue |
|
Mortality |
190 |
130 |
0.65 (0.52-0.81) |
35 |
0.00013 |
|
Mortality + all hospitalization |
507 |
425 |
0.76 (0.67-0.87) |
24 |
0.00004 |
|
Mortality + CV hospitalization |
395 |
314 |
0.73 (0.63-0.84) |
27 |
0.00002 |
|
Mortality + HF hospitalization |
357 |
271 |
0.69 (0.59-0.81) |
31 |
0.000004 |
Cardiovascular = CV; Heart failure = HF.
Figure 1. Survival Analysis for COPERNICUS (Intent-to-Treat)
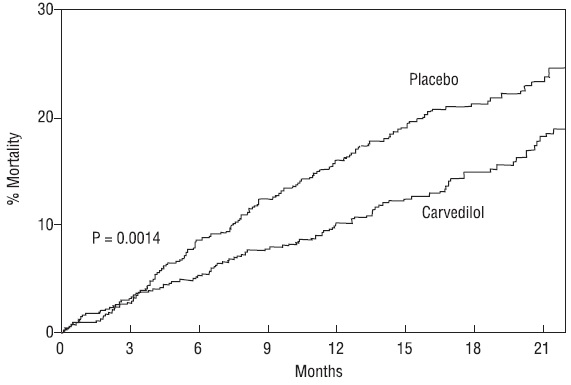
The effect on mortality was principally the result of a reduction in the rate of sudden death among subjects without worsening heart failure.
Patients’ global assessments, in which carvedilol-treated subjects were compared with placebo, were based on pre-specified, periodic patient self- assessments regarding whether clinical status post-treatment showed improvement, worsening or no change compared with baseline. Subjects treated with carvedilol showed significant improvements in global assessments compared with those treated with placebo in COPERNICUS.
The protocol also specified that hospitalizations would be assessed. Fewer subjects on carvedilol than on placebo were hospitalized for any reason (372 versus 432, P = 0.0029), for cardiovascular reasons (246 versus 314, P = 0.0003), or for worsening heart failure (198 versus 268, P = 0.0001).
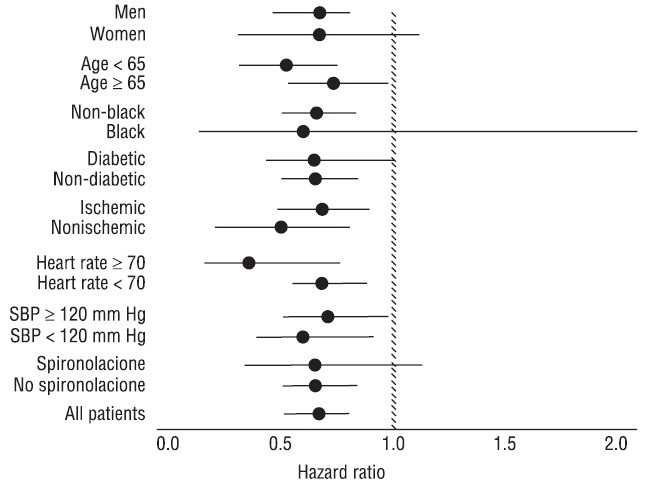
Carvedilol had a consistent and beneficial effect on all-cause mortality as well as the combined end points of all-cause mortality plus hospitalization (total, CV, or for heart failure) in the overall trial population and in all subgroups examined, including men and women, elderly and non-elderly, blacks and non-blacks, and diabetics and non-diabetics (see Figure 2).
Figure 2. Effects on Mortality for Subgroups in COPERNICUS
14.2 Left Ventricular Dysfunction following Myocardial Infarction
CAPRICORN was a double-blind trial comparing carvedilol and placebo in 1,959 subjects with a recent myocardial infarction (within 21 days) and left ventricular ejection fraction of less than or equal to 40%, with (47%) or without symptoms of heart failure. Subjects given carvedilol received 6.25 mg twice daily, titrated as tolerated to 25 mg twice daily. Subjects had to have a systolic blood pressure greater than 90 mm Hg, a sitting heart rate greater than 60 beats per minute, and no contraindication to β-blocker use. Treatment of the index infarction included aspirin (85%), IV or oral β-blockers (37%), nitrates (73%), heparin (64%), thrombolytics (40%), and acute angioplasty (12%). Background treatment included ACE inhibitors or angiotensin receptor blockers (97%), anticoagulants (20%), lipid-lowering agents (23%), and diuretics (34%). Baseline population characteristics included an average age of 63 years, 74% male, 95% Caucasian, mean blood pressure 121/74 mm Hg, 22% with diabetes, and 54% with a history of hypertension. Mean dosage achieved of carvedilol was 20 mg twice daily; mean duration of follow-up was 15 months.
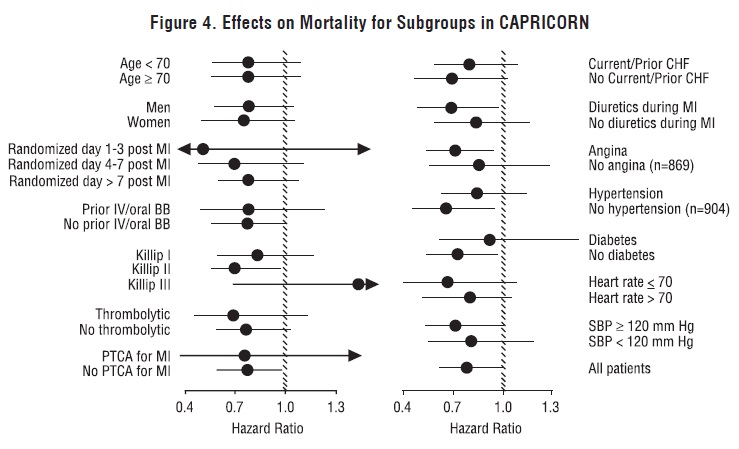
All-cause mortality was 15% in the placebo group and 12% in the carvedilol group, indicating a 23% risk reduction in subjects treated with carvedilol (95% CI 2% to 40%, P = 0.03), as shown in Figure 3. The effects on mortality in various subgroups are shown in Figure 4. Nearly all deaths were cardiovascular (which were reduced by 25% by carvedilol), and most of these deaths were sudden or related to pump failure (both types of death were reduced by carvedilol). Another trial end point, total mortality and all-cause hospitalization, did not show a significant improvement.
There was also a significant 40% reduction in fatal or non-fatal myocardial infarction observed in the group treated with carvedilol (95% CI 11% to 60%, P = 0.01). A similar reduction in the risk of myocardial infarction was also observed in a meta-analysis of placebo-controlled trials of carvedilol in heart failure.
Figure 3. Survival Analysis for CAPRICORN (Intent-to-Treat)
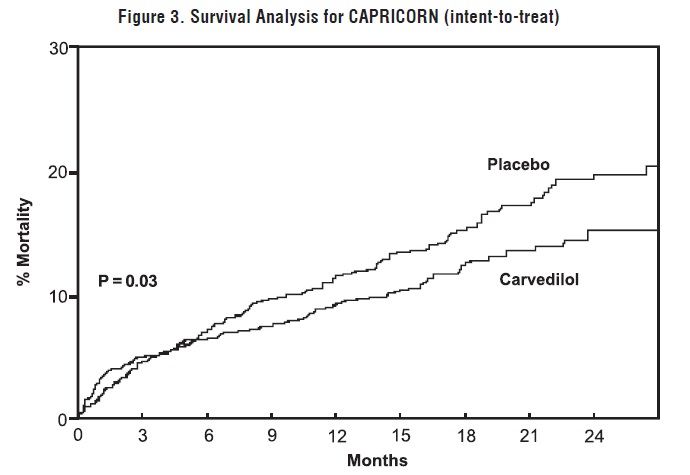
Figure 4. Effects on Mortality for Subgroups in CAPRICORN
14.3 Hypertension
Carvedilol was studied in 2 placebo-controlled trials that utilized twice- daily dosing, at total daily doses of 12.5 to 50 mg. In these and other trials, the starting dose did not exceed 12.5 mg. At 50 mg per day, carvedilol reduced sitting trough (12-hour) blood pressure by about 9/5.5 mm Hg; at 25 mg per day the effect was about 7.5/3.5 mm Hg. Comparisons of trough to peak blood pressure showed a trough to peak ratio for blood pressure response of about 65%. Heart rate fell by about 7.5 beats per minute at 50 mg per day. In general, as is true for other β-blockers, responses were smaller in black than non-black subjects. There were no age- or gender-related differences in response.
The peak antihypertensive effect occurred 1 to 2 hours after a dose. The dose- related blood pressure response was accompanied by a dose-related increase in adverse effects [see Adverse Reactions ( 6)].
14.4 Hypertension with Type 2 Diabetes Mellitus
In a double-blind trial (GEMINI), carvedilol, added to an ACE inhibitor or angiotensin receptor blocker, was evaluated in a population with mild-to- moderate hypertension and well controlled type 2 diabetes mellitus. The mean HbA1c at baseline was 7.2%. Carvedilol was titrated to a mean dose of 17.5 mg twice daily and maintained for 5 months. Carvedilol had no adverse effect on glycemic control, based on HbA1c measurements (mean change from baseline of 0.02%, 95% CI -0.06 to 0.10, P = NS) [see Warnings and Precautions ( 5.6)].
