ivermectin
These highlights do not include all the information needed to use IVERMECTIN CREAM safely and effectively. See full prescribing information for IVERMECTIN CREAM. IVERMECTIN cream, for topical useInitial U.S. Approval: 1996
6b4a7400-d34c-4947-90e6-340f7087a702
HUMAN PRESCRIPTION DRUG LABEL
Mar 27, 2024
Viona Pharmaceuticals Inc
DUNS: 081468959
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ivermectin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Ivermectin Cream, 1%
45 gm
NDC 72578-120-08
Rx only

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Ivermectin cream is indicated for the treatment of inflammatory lesions of rosacea.
Ivermectin cream is indicated for the treatment of inflammatory lesions of rosacea. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None.
None. (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During clinical trials, 2,047 subjects with inflammatory lesions of rosacea received ivermectin cream once daily. A total of 1,555 subjects were treated once daily for more than 12 weeks and 519 for approximately one year.
Adverse reactions, reported in ≤ 1% of subjects treated with ivermectin cream for at least 3 months in vehicle-controlled clinical trials, included skin burning sensation and skin irritation.
6.2 Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Local adverse reactions: contact dermatitis and allergic dermatitis.
In controlled clinical trials with ivermectin the most common adverse reactions (incidence ≤ 1 %) included skin burning sensation and skin irritation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Viona Pharmaceuticals Inc. at 1-888-304-5011 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
In vitro studies have shown that ivermectin cream, at therapeutic concentrations, neither inhibits nor induces cytochrome P450 (CYP450) enzymes.
SPL UNCLASSIFIED SECTION
Manufactured by:
Zydus Lifesciences Ltd.
Changodar, Ahmedabad, India
Distributed by:
Viona Pharmaceuticals Inc.
Cranford, NJ 07016
Rev.: 12/22
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Apply to the affected areas of the face once daily. Use a pea-size amount for each area of the face (forehead, chin, nose, each cheek) that is affected. Spread as a thin layer, avoiding the eyes and lips.
Ivermectin cream is not for oral, ophthalmic or intravaginal use.
- Apply to the affected areas once daily. (2)
- Not for oral, ophthalmic or intravaginal use. (2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Cream, 1%.
Each gram of ivermectin cream contains 10 mg of ivermectin, USP in a white to pale yellow homogeneous cream. Ivermectin cream is supplied in tubes of 30 g, 45 g and 60 g.
Cream, 1%, supplied in tubes of 30 g, 45 g and 60 g. (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There are no adequate and well-controlled studies in pregnant women. Ivermectin cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Note: The animal multiples of human exposure calculations were based on AUC comparisons. The maximum topical human dose (MTHD) of ivermectin cream is 1 g applied once daily.
Risk Summary
The available data on the use of ivermectin, including ivermectin cream, in pregnant women are insufficient to establish a drug- associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
In animal reproduction studies, ivermectin induced adverse developmental outcomes when orally administered to pregnant rats and rabbits during the period of organogenesis at doses 1909 or 354 times the maximum recommended human dose (MRHD), respectively. These orally administered doses were maternally toxic to pregnant rats and rabbits. In a pre-and postnatal developmental study in rats, neonatal toxicity and adverse effects on behavioral development were observed when ivermectin was orally administered to pregnant females during gestation and lactation (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
No adequate and well-controlled trials of ivermectin cream have been conducted in pregnant women. Retrospective observational studies evaluated pregnancy outcomes in over 700 women in various stages of pregnancy who received oral ivermectin for the treatment of soil-transmitted helminths in rural Africa. In an additional, randomized open-label trial, 397 pregnant women in their second trimester received a single dose of oral ivermectin, or ivermectin plus albendazole, for soil-transmitted helminths. When compared with a pregnant, untreated population, no differences in pregnancy outcomes were observed between the treated and untreated populations. These studies cannot definitively establish or exclude any drug-associated risk during pregnancy, because either the timing of administration during gestation was not accurately ascertained or the administration occurred only during the second trimester.
Animal Data
Systemic embryofetal development studies were conducted in rats and rabbits. Oral doses of 1.5, 4, and 12mg/kg/day ivermectin were administered during the period of organogenesis to pregnant female rats. Maternal death occurred at 12 mg/kg/day [1909 times the MRHD based on area under the curve (AUC) comparison]. Cleft palate occurred in the fetuses from the 12 mg/kg/day (1909 times the MRHD based on AUC comparison) group. No treatment related embryofetal toxicity or malformations were noted at 4 mg/kg/day (708 times the MRHD based on AUC comparison). Oral doses of 0.5, 1.5, 2.5, 3.5 and 4.5 mg/kg/day ivermectin were administered during the period of organogenesis to pregnant female rabbits. Maternal death occurred at doses ≥ 2.5 mg/kg/day (72 times the MRHD based on AUC comparison). Carpal flexure occurred in the fetuses from the 4.5 mg/kg/day (354 times the MRHD based on AUC comparison) group. Fetal weight decrease was noted at 3.5mg/kg/day (146 times the MRHD based on AUC comparison). No treatment related embryofetal toxicity or malformations were noted at 2.5 mg/kg/day (72 times the MRHD based on AUC comparison).
A pre-and postnatal development study was conducted in rats. Oral doses of 1, 2 and 4 mg/kg/day ivermectinwere administered to pregnant female rats during gestational days 6-20 and lactation days 2-20. Neonatal death occurred at doses ≥ 2 mg/kg/day. Behavior development of newborn rats was adversely affected at all doses.
8.2 Lactation
Risk Summary
The presence of ivermectin in human milk following topical administration of ivermectin has not been evaluated. There are no data available regarding the effects of ivermectin on milk production. Published literature suggests that ivermectin was detectable in human milk in 4 lactating women after a single 150 mcg/kg oral dose of ivermectin. However, there is insufficient information from this report to determine the effects of ivermectin on the breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ivermectin cream and any potential adverse effects on the breastfed infant from ivermectin cream or from the underlying maternal conditions.
8.4 Pediatric Use
Safety and effectiveness of ivermectin cream in pediatric patients have not been established.
8.5 Geriatric Use
Of the 1,371 subjects in the two pivotal clinical studies of ivermectin cream, 170 (12.4%) were 65 and over, while 37 (2.7%) were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
OVERDOSAGE SECTION
10 OVERDOSAGE
In accidental or significant exposure to unknown quantities of veterinary formulations of ivermectin in humans, either by ingestion, inhalation, injection or exposure to body surfaces, the following adverse effects have been reported most frequently: rash, edema, headache, dizziness, asthenia, nausea, vomiting and diarrhea. Other adverse effects that have been reported include: seizure, ataxia, dyspnea, abdominal pain, paresthesia, urticaria and contact dermatitis.
In case of accidental ingestion, supportive therapy, if indicated, should include parenteral fluids and electrolytes, respiratory support (oxygen and mechanical ventilation if necessary) and pressor agents if clinically significant hypotension is present. Induction of emesis and/or gastric lavage as soon as possible, followed by purgatives and other routine anti-poison measures, may be indicated if needed to prevent absorption of ingested material.
DESCRIPTION SECTION
11 DESCRIPTION
Ivermectin cream, 1% is a white to pale yellow homogeneous cream. Each gram of ivermectin cream contains 10 mg of ivermectin, USP. It is intended for topical use.
Ivermectin is a semi-synthetic derivative isolated from the fermentation of Streptomyces avermitilis that belongs to the avermectin family of macrocyclic lactones.
Ivermectin is a mixture containing not less than 95.0 % and not more than 102.0 % of 5-O-demethyl-22,23-dihydroavermectin A1a plus 5-O-demethyl-25-de(1-methylpropyl)-25-(1-methylethyl)-22,23-dihydroavermectin A1a, generally referred to as 22,23-dihydroavermectin B1a and B1b or H2B1a and H2B1b, respectively; and the ratio (calculated by area percentage) of component H2B1a/(H2B1a + H2B1b)) is not less than 90.0 %.
The respective empirical formulas of H2B1a and H2B1b are C48H74O14 and C47H72O14 with molecular weights of 875.10 and 861.07 respectively.
The structural formulas are:
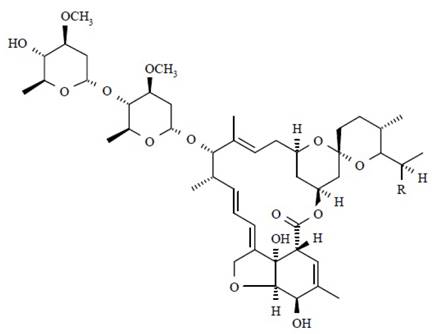
Component H2B1a: R = C2H5, Component H2B1b: R = CH3.
Ivermectin cream contains the following inactive ingredients: carbomer copolymer type B, cetyl alcohol, citric acid monohydrate, cocodiethanolamide, dimethicone, dimethyl isosorbide, edetate disodium, glycerin, isopropyl palmitate, methyl paraben, propyl paraben, purified water, sodium hydroxide, sodium lauryl sulfate, and stearyl alcohol.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2 year dermal mouse carcinogenicity study, ivermectin was administered to CD-1 mice at topical doses of 1 mg/kg/day, 3 mg/kg/day and 10 mg/kg/day (0.1%, 0.3% and 1% ivermectin cream applied at 2 ml/kg/day). No drug-related tumors were noted in this study up to the highest dose evaluated in this study of 10 mg/kg/day (747 times the MRHD based on AUC comparison).
In a 2 year oral rat carcinogenicity study, ivermectin was administered to Wistar rats at gavage doses of 1 mg/kg/day, 3 mg/kg/day and 9 mg/kg/day. A statistically significant increase in the incidence of hepatocellular adenoma was noted in males treated with 9 mg/kg/day (1766 times the MRHD based on AUC comparison) ivermectin. The clinical relevance of this finding is unknown. No drug-related tumors were noted in females up to the highest dose evaluated in this study of 9 mg/kg/day (1959 times the MRHD based on AUC comparison). No drug-related tumors were noted in males at doses ≤ 3 mg/kg/day (599 times the MRHD based on AUC comparison).
Ivermectin revealed no evidence of genotoxic potential based on the results of two in vitro genotoxicity tests (the Ames test and the L5178Y/TK+/-mouse lymphoma assay) and one in vivo genotoxicity test (rat micronucleus assay).
In a fertility study, oral doses of 0.1 mg/kg/day, 1 mg/kg/day and 9 mg/kg/day ivermectin were administered to male and female rats. Mortality occurred at 9 mg/kg/day (1027 times the MRHD based on AUC comparison). The precoital period was generally prolonged at 9 mg/kg/day. No treatment related effects on fertility or mating performance were noted at doses ≤ 1 mg/kg/day (68 times the MRHD based on AUC comparison).
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
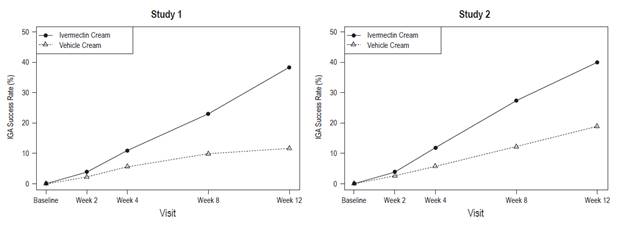
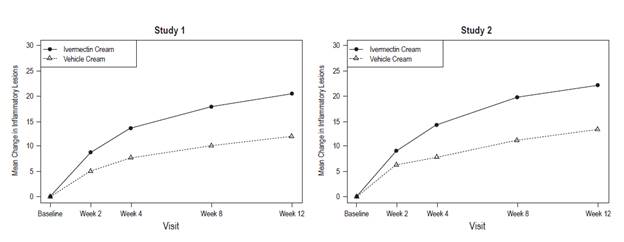
Ivermectin cream applied once daily at bedtime was evaluated in the treatment of inflammatory lesions of rosacea in two randomized, double-blind, vehicle- controlled clinical trials, which were identical in design. The trials were conducted in 1,371 subjects aged 18 years and older who were treated once daily for 12 weeks with either ivermectin cream or vehicle cream.
Overall, 96% of subjects were Caucasian and 67% were female. Using the 5-point Investigator Global Assessment (IGA) scale (0=clear, 1=almost clear, 2=mild, 3=moderate, 4=severe), 79% of subjects were scored as moderate (IGA=3) and 21% scored as severe (IGA= 4) at baseline.
The co-primary efficacy endpoints in both pivotal trials were the success rate based on the IGA outcome (percentage of subjects "clear" and "almost clear") and absolute change from baseline in inflammatory lesion counts at Week 12. Table 1 presents the co-primary efficacy results at Week 12. Ivermectin cream was more effective than vehicle cream on the co-primary efficacy endpoints starting from 4 weeks of treatment in both studies, see Figures 1 through 4.
Table 1 Co-Primary Efficacy Results at Week 12|
** Study 1** |
** Study 2** | |||
|
** Ivermectin Cream** |
** Vehicle Cream (N=232)** |
** Ivermectin Cream** |
** Vehicle Cream (N=229)** | |
|
** Investigator Global Assessment:** | ||||
|
Number (%) of Subjects Clear or Almost Clear |
173 |
27 |
184 |
43 |
|
** Inflammatory Lesion Counts:** | ||||
|
Mean Absolute (%) Change from Baseline |
20.5 |
12 |
22.2 |
13.4 |
Figures 1 and 2:
IGA Success Rates Over Time
Figures 3 and 4:
Mean Absolute Change in Inflammatory Lesion Counts from Baseline Over Time
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Ivermectin cream, 1% is a white to pale yellow homogeneous cream, supplied in a laminated tube with a child-resistant cap in the following sizes:
30 gram NDC 72578-120-06
45 gram NDC 72578-120-08
60 gram NDC 72578-120-02
Storage
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Patients using ivermectin cream should receive the following instruction:
Keep this and all drugs out of the reach of children.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please address medical inquiries to, drugsafety@vionausa.com or Tel.: 1-888-304-5011.
SPL PATIENT PACKAGE INSERT SECTION
SPL PATIENT PACKAGE INSERT
|
** PATIENT INFORMATION** |
|
** Important: Ivermectin cream is for use on the skin only (topical use).** Do not use ivermectin cream in your mouth, eyes, or vagina. |
|
** What is ivermectin cream?** ivermectin cream is a prescription medicine used on the skin (topical) to treat pimples and bumps (inflammatory lesions) caused by a condition called rosacea. It is not known if ivermectin cream is safe and effective in children. |
|
** Before using ivermectin cream, tell your healthcare provider about all your medical conditions, including if you:** • are pregnant or plan to become pregnant. It is not known if ivermectin cream will harm your unborn baby. • are breastfeeding or plan to breastfeed. It is not known if ivermectin cream passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you use ivermectin cream.** Tell your healthcare provider about all of the medicines you take** , including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|
** How should I use ivermectin cream? See the detailed "Instructions for Use" that comes with ivermectin cream for information on how to apply ivermectin cream.** • Use ivermectin cream exactly as your healthcare provider tells you to. • Apply ivermectin cream to the affected areas of your face 1 time a day. • Avoid contact with your eyes and lips • If ivermectin cream is accidentally swallowed (ingested), call your healthcare provider or go to the nearest hospital emergency room right away. |
|
** What are the possible side effects of ivermectin cream? The most common side effects of ivermectin cream include** skin burning sensation and skin irritation. These are not all of the possible side effects of ivermectin cream. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Galderma Laboratories, L.P. at 1-866-735-41 |
|
** How should I store ivermectin cream?** • Store ivermectin cream at room temperature between 68°F to 77°F (20°C to 25°C).** Keep ivermectin cream and all medicines out of the reach of children.** |
|
** General information about the safe and effective use of ivermectin cream. ** Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ivermectin cream for a condition for which it was not prescribed. Do not give ivermectin cream to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ivermectin cream that is written for health professionals |
|
** What are the ingredients in ivermectin cream? Active ingredient:** ivermectin** Inactive ingredients:** carbomer copolymer type B, cetyl alcohol, citric acid monohydrate, dimethicone, edetate disodium, glycerin, isopropyl palmitate, methylparaben, oleyl alcohol, phenoxyethanol, polyoxyl 20 cetostearyl ether, propylene glycol, propylparaben, purified water, sodium hydroxide, sorbitan monostearate, and stearyl alcohol. |
|
** Manufactured by:** ** Distributed by:** |
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of ivermectin cream in treating rosacea lesions is unknown.
12.2 Pharmacodynamics
Cardiac Electrophysiology
At therapeutic doses, ivermectin cream is not expected to prolong QTc interval.
12.3 Pharmacokinetics
Absorption
The absorption of ivermectin from ivermectin cream was evaluated in a clinical trial in 15 adult male and female subjects with severe papulopustular rosacea applying 1 g ivermectin cream, 1% once daily. At steady state (after 2 weeks of treatment), the highest mean ± standard deviation) plasma concentrations of ivermectin peaked (Tmax) at 10 hours ± 8 hours post-dose, the maximum concentration (Cmax) was 2.10 ng/mL ± 1.04 ng/mL (range: 0.69 ng/mL to 4.02 ng/mL) and the area under the concentration curve (AUC0-24hr) was 36.14 ng.hr/mL ± 15.56 ng.hr/mL (range: 13.69 ng.hr/mL to 75.16 ng.hr/mL). In addition, systemic exposure assessment in longer treatment duration (Phase 3 studies) showed that there was no plasma accumulation of ivermectin over the 52 week treatment period.
Distribution
An in vitro study demonstrated that ivermectin is greater than 99% bound to plasma proteins and is bound primarily to human serum albumin. No significant binding of ivermectin to erythrocytes was observed.
Metabolism
In vitro studies using human hepatic microsomes and recombinant CYP450 enzymes have shown that ivermectin is primarily metabolized by CYP3A4. In vitro studies show that ivermectin at therapeutic concentrations does not inhibit the CYP450 isoenzymes 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4 or 4A11 or induce 1A2, 2B6, 2C9 or 3A4.
Excretion
The apparent terminal half-life averaged 6.5 days (mean ± standard deviation: 155 hours ± 40 hours, range 92 hours to 238 hours) in patients receiving a once daily cutaneous application of ivermectin cream for 28 days.
