Warfarin Sodium
These highlights do not include all the information needed to use WARFARIN SODIUM TABLETS safely and effectively. See full prescribing information for WARFARIN SODIUM TABLETS. WARFARIN SODIUM tablets, for oral useInitial U.S. Approval: 1954
a1860448-d4ef-40f2-b096-056429ca3aea
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Warfarin 1mg Tablet

DESCRIPTION SECTION
11 DESCRIPTION
Warfarin sodium tablets, USP contain warfarin sodium, an anticoagulant that acts by inhibiting vitamin K-dependent coagulation factors. The chemical name of warfarin sodium is 3-(α-acetonylbenzyl)-4-hydroxycoumarin sodium salt, which is a racemic mixture of the R- and S-enantiomers. Crystalline warfarin sodium is an isopropanol clathrate. Its structural formula may be represented as follows:
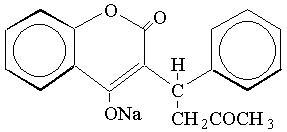
C19H15NaO4 M.W. 330.31
Crystalline warfarin sodium occurs as a white, odorless, crystalline powder that is discolored by light. It is very soluble in water, freely soluble in alcohol, and very slightly soluble in chloroform and ether.
Each tablet, for oral administration, contains 1 mg, 2 mg, 2.5 mg, 3 mg, 4 mg, 5 mg, 6 mg, 7.5 mg or 10 mg warfarin sodium, USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose.
The 1 mg also contains FD&C red no. 40.
The 2 mg also contains FD&C blue no. 2 aluminum lake and FD&C red no. 40 aluminum lake.
The 2.5 mg also contains D&C yellow no. 10 aluminum lake and FD&C blue no. 1 aluminum lake.
The 3 mg also contains FD&C yellow no. 6 aluminum lake, FD&C blue no. 2 aluminum lake, FD&C red no. 40 aluminum lake, and D&C yellow no. 10 aluminum lake.
The 4 mg also contains FD&C blue no. 1 aluminum lake and FD&C blue no. 2 aluminum lake.
The 5 mg also contains FD&C yellow no. 6 aluminum lake, FD&C red no. 40 aluminum lake and D&C yellow no. 10 aluminum lake.
The 6 mg also contains D&C yellow no. 10 aluminum lake and FD&C blue no. 1 aluminum lake.
The 7.5 mg also contains D&C yellow no. 10 aluminum lake and FD&C yellow no. 6 aluminum lake.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Warfarin sodium tablets USP, 1 mg are available as pink, capsule-shaped, biconvex scored tablets, debossed with TV/1 on the scored side and 1712 on the other side containing 1 mg warfarin sodium, USP.
NDC: 71335-0270-9: 10 Tablets in a BOTTLE
NDC: 71335-0270-1: 30 Tablets in a BOTTLE
NDC: 71335-0270-2: 60 Tablets in a BOTTLE
NDC: 71335-0270-3: 100 Tablets in a BOTTLE
NDC: 71335-0270-4: 50 Tablets in a BOTTLE
NDC: 71335-0270-5: 90 Tablets in a BOTTLE
NDC: 71335-0270-6: 21 Tablets in a BOTTLE
NDC: 71335-0270-7: 15 Tablets in a BOTTLE
NDC: 71335-0270-8: 20 Tablets in a BOTTLE
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Protect from light. Keep tightly closed.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Special Handling
Procedures for proper handling and disposal of potentially hazardous drugs should be considered. Guidelines on this subject have been published [see References (15)].
Pharmacy and clinical personnel who are pregnant should avoid exposure to crushed or broken tablets [see Use in Specific Populations (8.1)].
Keep this and all medications out of the reach of children.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
