Vascepa
These highlights do not include all the information needed to use VASCEPA safely and effectively. See full prescribing information for VASCEPA. VASCEPA (icosapent ethyl) capsules, for oral use Initial U.S. Approval: 2012
9c1a2828-1583-4414-ab22-a60480e8e508
HUMAN PRESCRIPTION DRUG LABEL
Apr 24, 2023
Amarin Pharma Inc.
DUNS: 896179731
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
icosapent ethyl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
icosapent ethyl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
icosapent ethyl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
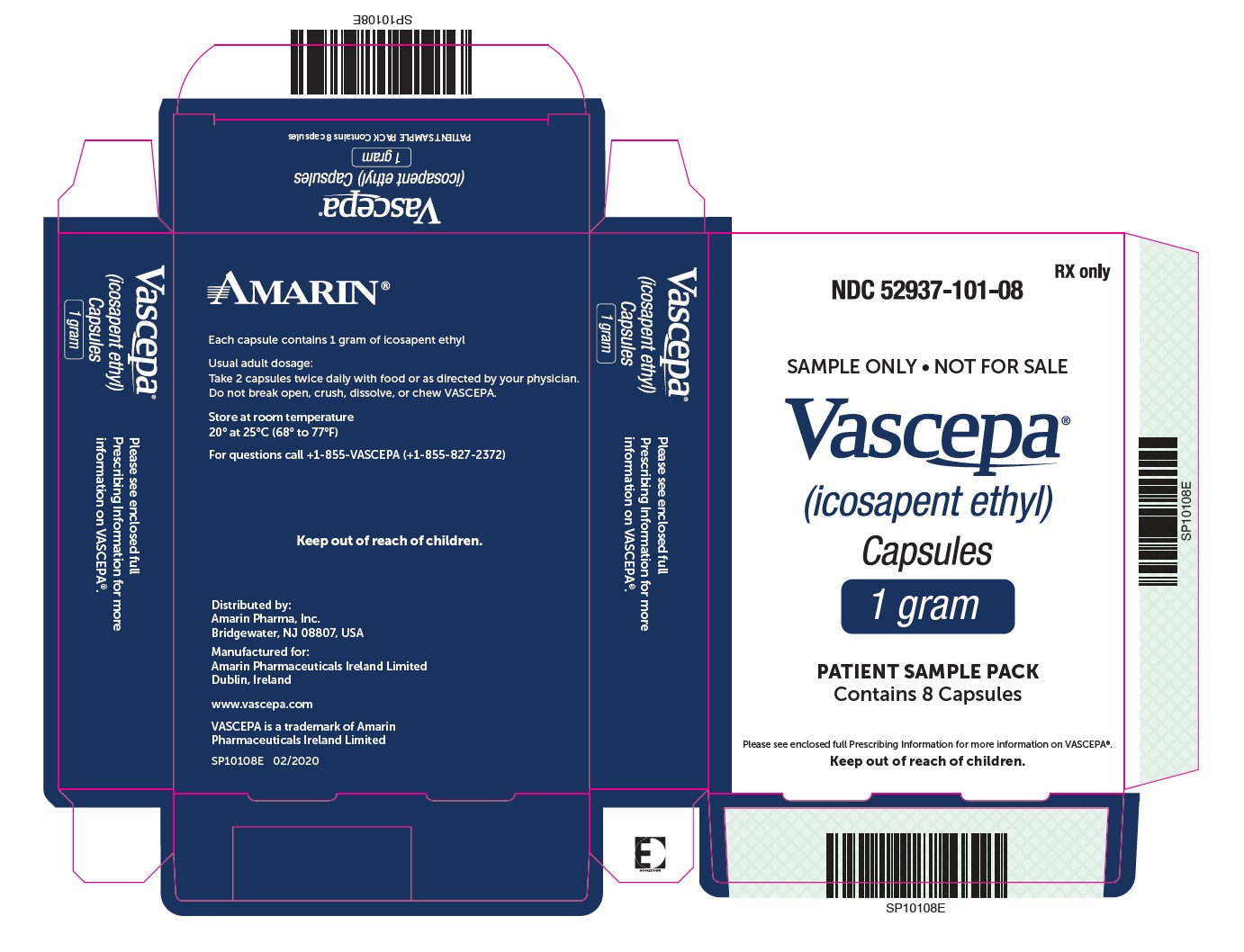
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 8 Capsule Blister Carton
RX only
NDC 52937-101-08
SAMPLE ONLY • NOT FOR SALE
Vascepa®
(icosapent ethyl)
Capsules
1 gram
PATIENT SAMPLE PACK
Contains 8 Capsules
Please see enclosed full Prescribing Information for more information on
VASCEPA®.
Keep out of reach of children.
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Atrial Fibrillation/Flutter
VASCEPA is associated with an increased risk of atrial fibrillation or atrial flutter requiring hospitalization. In a double-blind, placebo-controlled trial of 8,179 statin-treated subjects with established cardiovascular disease (CVD) or diabetes plus an additional risk factor for CVD, adjudicated atrial fibrillation or atrial flutter requiring hospitalization for 24 or more hours occurred in 127 (3%) patients treated with VASCEPA compared to 84 (2%) patients receiving placebo [HR= 1.5 (95% CI 1.14, 1.98)]. The incidence of atrial fibrillation was greater in patients with a previous history of atrial fibrillation or atrial flutter.
5.2 Potential for Allergic Reactions in Patients with Fish Allergy
VASCEPA contains ethyl esters of the omega-3 fatty acid, eicosapentaenoic acid (EPA), obtained from the oil of fish. It is not known whether patients with allergies to fish and/or shellfish are at increased risk of an allergic reaction to VASCEPA. Inform patients with known hypersensitivity to fish and/or shellfish about the potential for allergic reactions to VASCEPA and advise them to discontinue VASCEPA and seek medical attention if any reactions occur.
5.3 Bleeding
VASCEPA is associated with an increased risk of bleeding. In a double-blind, placebo-controlled cardiovascular outcomes trial of 8,179 patients, 482 (12%) patients receiving VASCEPA experienced a bleeding event compared to 404 (10%) patients receiving placebo. Serious bleeding events occurred in 111 (3%) of patients on VASCEPA vs. 85 (2%) of patients receiving placebo. The incidence of bleeding was greater in patients receiving concomitant antithrombotic medications, such as aspirin, clopidogrel, or warfarin.
Atrial Fibrillation/Flutter: VASCEPA was associated with an increased risk of atrial fibrillation or atrial flutter requiring hospitalization in a double- blind, placebo-controlled trial. The incidence of atrial fibrillation was greater in patients with a previous history of atrial fibrillation or atrial flutter. (5.1)
Potential for Allergic Reactions in Patients with Fish Allergy: VASCEPA contains ethyl esters of the omega-3 fatty acid, eicosapentaenoic acid (EPA), obtained from the oil of fish. It is not known whether patients with allergies to fish and/or shellfish are at increased risk of an allergic reaction to VASCEPA. Inform patients with known hypersensitivity to fish and/or shellfish about the potential for allergic reactions and advise them to discontinue VASCEPA and seek medical attention if any reactions occur. (5.2)
Bleeding: VASCEPA was associated with an increased risk of bleeding in a double-blind, placebo-controlled trial. The incidence of bleeding was greater in patients receiving concomitant antithrombotic medications, such as aspirin, clopidogrel, or warfarin. (5.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Atrial Fibrillation or Atrial Flutter [see Warnings and Precautions (5.1)]
- Potential for Allergic Reactions in Patients with Fish Allergy [see Warnings and Precautions (5.2)]
- Bleeding [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Cardiovascular Outcomes Trial
In a double-blind, randomized, placebo-controlled cardiovascular outcomes trial, 8,179 statin-stabilized patients were randomized to receive VASCEPA or placebo and followed for a median of 4.9 years [see Clinical Studies (14.1)]. The median age at baseline was 64 years, 29% were women, 90% White, 5% Asian, 2% were Black, and 4% identified as Hispanic ethnicity.
Common adverse reactions (incidence ≥3% on VASCEPA and ≥1% more frequent than placebo) included musculoskeletal pain, peripheral edema, constipation, gout, and atrial fibrillation.
Hypertriglyceridemia Trials
In two randomized, double-blind, placebo-controlled trials in patients with triglyceride levels between 200 and 2000 mg/dL treated for 12 weeks, adverse reactions reported with VASCEPA at an incidence ≥1% more frequent than placebo based on pooled data included arthralgia and oropharyngeal pain.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during post-approval use of VASCEPA. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Diarrhea
- Blood triglycerides increased
- Abdominal discomfort
- Pain in the extremities
Common adverse reactions in the cardiovascular outcomes trial (incidence ≥3% and ≥1% more frequent than placebo): musculoskeletal pain, peripheral edema, constipation, gout, and atrial fibrillation (6.1)
Common adverse reactions in the hypertriglyceridemia trials (incidence ≥1% more frequent than placebo): arthralgia and oropharyngeal pain. (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contact Amarin Pharma, Inc. at 1-855-VASCEPA (1-855-827-2372) or contact the FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year rat carcinogenicity study with oral gavage doses of 0.09, 0.27, and 0.91 g/kg/day icosapent ethyl, respectively, males did not exhibit drug- related neoplasms. Hemangiomas and hemangiosarcomas of the mesenteric lymph node, the site of drug absorption, were observed in females at clinically relevant exposures based on body surface area comparisons across species relative to the maximum clinical dose of 4 g/day. Overall incidence of hemangiomas and hemangiosarcomas in all vascular tissues did not increase with treatment.
In a 6-month carcinogenicity study in Tg.rasH2 transgenic mice with oral gavage doses of 0.5, 1, 2, and 4.6 g/kg/day icosapent ethyl, drug-related incidences of benign squamous cell papilloma in the skin and subcutis of the tail was observed in high dose male mice. The papillomas were considered to develop secondary to chronic irritation of the proximal tail associated with fecal excretion of oil and therefore not clinically relevant. Drug-related neoplasms were not observed in female mice.
Icosapent ethyl was not mutagenic with or without metabolic activation in the bacterial mutagenesis (Ames) assay or in the in vivo mouse micronucleus assay. A chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells was positive for clastogenicity with and without metabolic activation.
In an oral gavage rat fertility study, ethyl-EPA, administered at doses of 0.3, 1, and 3 g/kg/day to male rats for 9 weeks before mating and to female rats for 14 days before mating through day 7 of gestation, increased anogenital distance in female pups and increased cervical ribs were observed at 3 g/kg/day (7 times human systemic exposure with 4 g/day clinical dose based on a body surface area comparison).
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
VASCEPA (icosapent ethyl) capsules are supplied as
|
Strength |
Quantity |
Description |
NDC |
|
0.5 gram capsules |
Bottles of 240 |
amber-colored soft-gelatin capsules imprinted with V500 |
52937-003-40 |
|
1 gram capsules |
Bottles of 120 |
amber-colored soft-gelatin capsules imprinted with VASCEPA |
52937-001-20 |
Store at 20° to 25° C (68° to 77°F); excursions permitted to 15° to 30° C (59° to 86°F) [see USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling before starting VASCEPA (Patient Information).
Inform patients that VASCEPA may increase their risk for atrial fibrillation or atrial flutter [see Warnings and Precautions (5.1)].
Inform patients with known hypersensitivity to fish and/or shellfish about the potential for allergic reactions to VASCEPA and advise them to discontinue VASCEPA and seek medical attention if any reactions occur [see Warnings and Precautions (5.2)].
Inform patients that VASCEPA may increase their risk for bleeding, especially if they are receiving other antithrombotic agents [see Warnings and Precautions (5.3)].
Advise patients to swallow VASCEPA capsules whole. Do not break open, crush, dissolve, or chew VASCEPA [see Dosage and Administration (2.2)].
Instruct patients to take VASCEPA as prescribed. If a dose is missed, patients should take it as soon as they remember. However, if they miss one day of VASCEPA, they should not double the dose when they take it.
For more information about VASCEPA, go to www.VASCEPA.com or call 1-855-VASCEPA (1-855-827-2372).
