Polyethylene Glycol 3350
Polyethelene Glycol 3350 Powder for OralSolution
3729a1d2-4c98-dfdf-e063-6394a90a5a87
HUMAN OTC DRUG LABEL
Jun 9, 2025
Kesin Pharma Corporation
DUNS: 117447816
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Polyethylene Glycol 3350
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
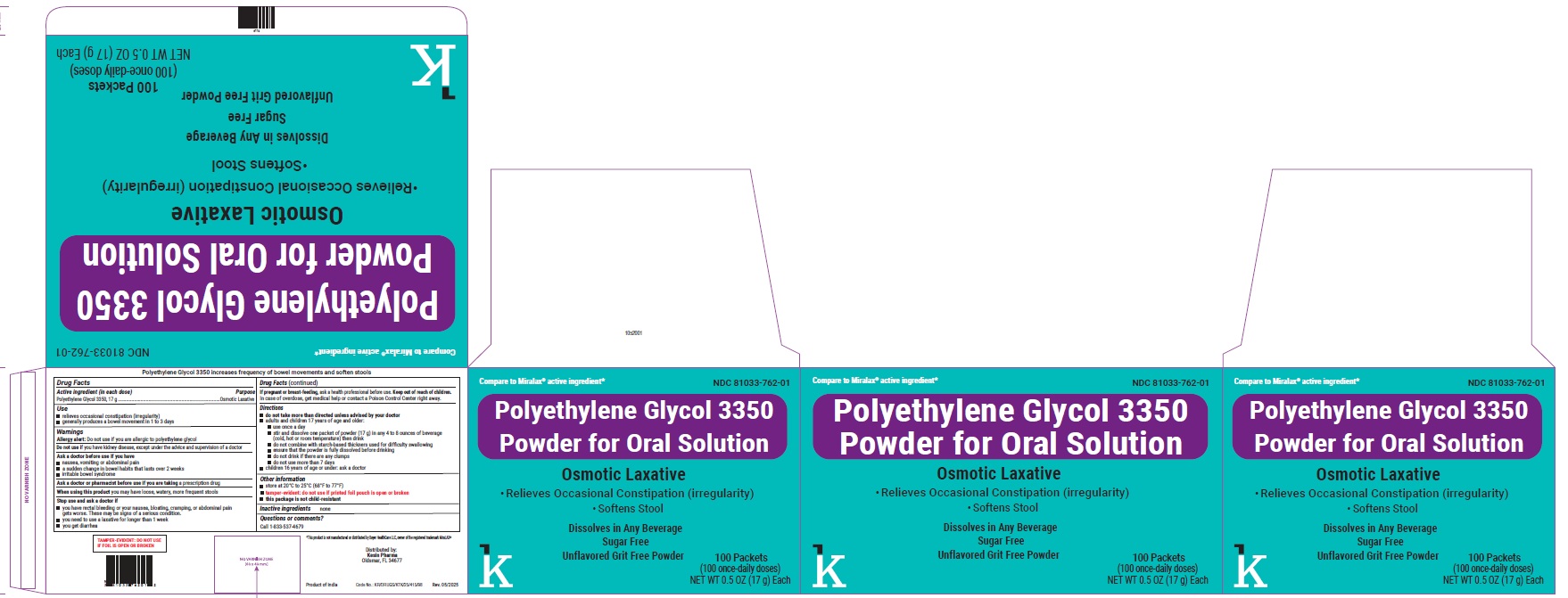
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 81033-762-01
Polyethylene Glycol 3350 Powder for Oral Solution
Osmotic laxative
100 Packets
(100 once-daily doses)
INDICATIONS & USAGE SECTION
Use
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 1 to 3 days
SPL UNCLASSIFIED SECTION
|
TAMPER-EVIDENT: DO NOT USE IF FOIL IS OPEN OR BROKEN |
*This product is not manufactured by Bay HealthCare LLC, owner of the registered trademark MiraLAX ®
Distributed by:
Kesin Pharma
Oldsmar, FL 34677

OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each dose)
Polyethlene Glycol 3350, 17 g
OTC - PURPOSE SECTION
Purpose
Osmotic Laxative
WARNINGS SECTION
Warnings
Allergy alert:
Do not use if you are allergic to polyethylene glycol
Do not use
if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
Ask a doctor or pharmacist before use if you are taking
a prescription drug
When using this product
you may have loose, watery, more frequent stools
Stop use and ask a doctor if
- you have rectal bleeding or your nausea, bloating, cramping or abdominal pain gets worse. These may be signs of a serious condition.
- you need to use a laxative for longer than 1 week
- you get diarrhea
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of the reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
*do not take more than directed unless advised by your doctor
- adults and children 17 yearsof age and older:
- use once a day
- stir and dissolve one packet of powder (17 g) in any 4 to 8 ounce of beverage (cold, hot or room temperature) then drink
- do not combine with starch-based thickners used for difficulty swallowing
- ensure that the powder is fully dissolved before drinking
- do not drink if there are any clumps
- do not use more than 7 days
- children 16 years of age or under: ask a doctor
OTHER SAFETY INFORMATION
Other information
- store at 20°C to 25°C (68°F to 77°F) *tamper-evident: do not use if printed foil pouch is open or broken *this package is not child-resistant
INACTIVE INGREDIENT SECTION
Inactive ingredients
none
OTC - QUESTIONS SECTION
Questions or comments?
Call 1-833-537-4679
