Haemonetics Anticoagulant Sodium Citrate
Haemonetics Anticoagulant Sodium Citrate
447528e6-55cd-4a9b-868e-2a741b2693d3
HUMAN PRESCRIPTION DRUG LABEL
Feb 2, 2021
Haemonetics Corporation
DUNS: 057827420
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
TRISODIUM CITRATE DIHYDRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Product Labeling

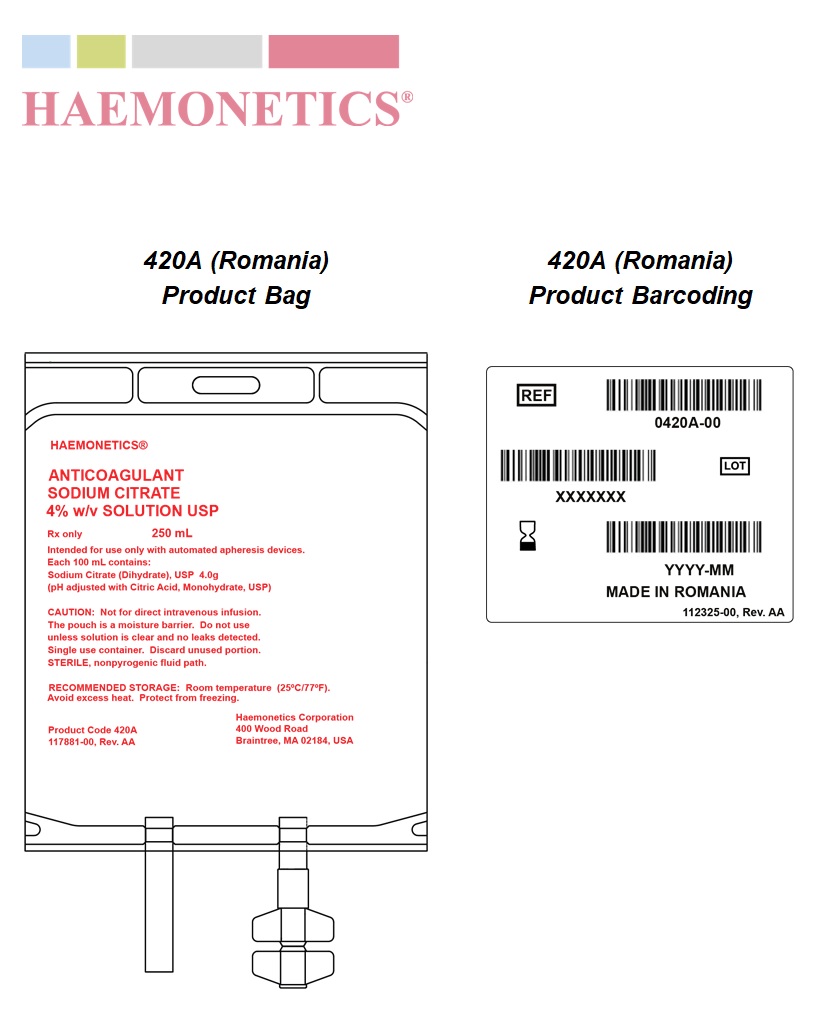

SPL UNCLASSIFIED SECTION
STERILE, nonpyrogenic fluid path.
WARNINGS SECTION
CAUTION:
Not for direct intravenous infusion. The pouch is a moisture barrier. Do not use unless solution is clear and no leaks detected. Single use container. Discard unused portion.
STORAGE AND HANDLING SECTION
RECOMMENDED STORAGE:
Room temperature (25°C/77°F). Avoid excess heat. Protect from freezing.
INFORMATION FOR PATIENTS SECTION
Product code
Product Code 420A
117881-00, Rev. AA-XXX
Haemonetics Corporation
400 Wood Road
Braintree, MA 02184 USA
