Fomepizole
Rx only Sterile Caution : Must be diluted prior to use. Do not use polycarbonate syringes or polycarbonate-containing needles (including polycarbonate filter needles) with fomepizole injection.
256910fe-91f2-48f6-b0b4-55edc52dacd4
HUMAN PRESCRIPTION DRUG LABEL
Feb 10, 2023
Zydus Pharmaceuticals USA Inc.
DUNS: 156861945
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Fomepizole
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Fomepizole Injection is a competitive inhibitor of alcohol dehydrogenase. The chemical name of fomepizole is 4-methylpyrazole. It has the molecular formula C4H6N2 and a molecular weight of 82.1. The structural formula is:
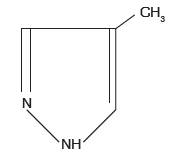
It is a clear to yellow liquid at room temperature. Its melting point is 25°C(77°F) and it may present as solid form at room temperature. Fomepizole is soluble in water and very soluble in ethanol, diethyl ether, and chloroform. Each vial contains 1.5 mL (1 g/mL) of fomepizole.
