GAMROZYNE
GAMROZYNE (gamithromycin)
e3038f84-55e5-4633-aa7c-5b750c3a716c
PRESCRIPTION ANIMAL DRUG LABEL
Sep 3, 2025
Bimeda, Inc
DUNS: 060492923
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
gamithromycin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
As with all drugs, the use of GAMROZYNE is contraindicated in animals previously found to be hypersensitive to this drug.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Transient animal discomfort and mild to moderate injection site swelling may
be seen in cattle treated with GAMROZYNE.
SPL UNCLASSIFIED SECTION
Approved by FDA under ANADA # 200-819
GAMROZYNE is a trademark of Bimeda, Inc.
Manufactured for:
Bimeda, Inc.
Le Sueur, MN 56058
Made in Canada
05/25
DESCRIPTION SECTION
DESCRIPTION
GAMROZYNE Injection for Cattle is a ready to use sterile parenteral solution
containing gamithromycin, a macrolide sub-class, 7a-azalide antimicrobial.
Each mL of GAMROZYNE contains 150 mg of gamithromycin as the free base, 1 mg
of monothioglycerol and 40 mg of succinic acid in a glycerol formal vehicle.
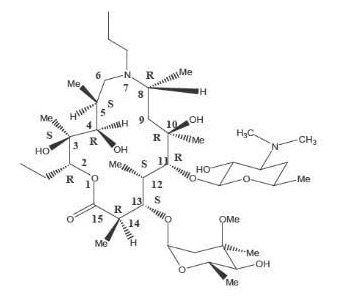
The chemical name of gamithromycin is
1-Oxa-7-azacyclopentadecan-15-one,13-[(2,6-dideoy-3-C-methyl-3-O-methyl-alpha- L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy
3,5,8,10,12,14-hexamethyl-7-propyl-11-{[3,4,6-trideoxy-3-(dimethylamino)-beta- D-xylo-hexopyranosyl]oxy}-, [(2R*,3S*,4R*,5S*,8R*,10R*, 11R*,12S*,13S*,14R*)]-and the structure is shown below.
VETERINARY INDICATIONS SECTION
INDICATIONS
GAMROZYNE is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis in beef and non-lactating dairy cattle. GAMROZYNE is also indicated for the control of respiratory disease in beef and non- lactating dairy cattle at high risk of developing BRD associated with Mannheimia haemolytica and Pasteurella multocida.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Administer GAMROZYNE one time as a subcutaneous injection in the neck at 6 mg/kg (2 mL/110 lb) body weight (BW). If the total dose exceeds 10 mL, divide the dose so that no more than 10 mL is administered at each injection site.
|
Body Weight (lb) |
** Dose Volume (mL)** |
|
110 |
2 |
|
220 |
4 |
|
330 |
6 |
|
440 |
8 |
|
550 |
10 |
|
660 |
12 |
|
770 |
14 |
|
880 |
16 |
|
990 |
18 |
|
1100 |
20 |
Animals should be appropriately restrained to achieve the proper route of administration. Use sterile equipment. Inject under the skin in front of the shoulder (see ILLUSTRATION).

WARNINGS SECTION
WARNING:
FOR USE IN CATTLE ONLY.
NOT FOR USE IN HUMANS.
KEEP THIS AND ALL DRUGS OUT OF REACH OF CHILDREN.
NOT FOR USE IN CHICKENS OR TURKEYS.
CONTACT INFORMATION
The Safety Data Sheet (SDS) contains more detailed occupational safety information. To report suspected adverse drug events, for technical assistance, or to obtain a copy of the SDS, contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or online at www.fda.gov/reportanimalae
RESIDUE WARNING SECTION
RESIDUE WARNINGS
Do not treat cattle within 35 days of slaughter. Because a discard time in milk has not been established, do not use in female dairy cattle 20 months of age or older. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal.
PRECAUTIONS SECTION
PRECAUTIONS
The effects of GAMROZYNE on bovine reproductive performance, pregnancy, and lactation have not been determined. Subcutaneous injection of GAMROZYNE may cause a transient local tissue reaction in some cattle that may result in trim loss of edible tissues at slaughter.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
The macrolide antimicrobials as a class are weak bases and as such concentrate in some cells (such as pulmonary leukocytes). Prolonged exposure of extracellular pulmonary pathogens to macrolides appears to reflect the slow release of drug from its intracellular reservoir to the site of action, the pulmonary epithelial lining fluid (ELF). It is the ELF that is relevant to the successful treatment and control of BRD. Gamithromycin is primarily bacteriostatic at therapeutic concentrations. However, in vitro bactericidal activity has been observed at concentrations of 10 μg/mL (Mueller-Hinton broth) and after exposure to the 6-hour and 24-hour plasma samples derived from cattle dosed at 6 mg gamithromycin/kg BW.
Macrolides typically exhibit substantially higher concentrations in the alveolar macrophages and ELF as compared to concentrations observed in plasma. Gamithromycin concentrations in the ELF and ELF cells exceed the concentrations observed in the plasma. Postmortem gamithromycin concentrations in ELF exceed the MIC90 of M. haemolytica, H. somni and P. multocida through at least 72 hours after drug administration. Because M. haemolytica, P. multocida and H. somni are extracellular pathogens, drug concentrations in the ELF are considered to be clinically relevant. The postmortem area under the concentration-time curve (AUC) observed in lysed ELF cells (e. g., alveolar macrophages) are at least 300-times greater than that in the plasma. Although published studies suggest that inflammation can increase the release of drug from macrophages and neutrophils, these high concentrations in the alveolar macrophages should not be considered indicative of the magnitude or duration of response to the pathogens for which this product is indicated.
Gamithromycin administered subcutaneously in the neck of cattle at a single dosage of 6 mg/kg BW is rapidly and completely absorbed, with peak concentrations generally occurring within 1 hour after administration. Based upon plasma and lung homogenate data, the terminal half-life (T½) of gamithromycin is approximately 3 days. In vitro plasma protein binding studies show that 26% of the gamithromycin binds to plasma protein, resulting in free drug available for rapid and extensive distribution into body tissues. The free drug is rapidly cleared from the systemic circulation with a clearance rate of 712 mL/hr/kg and a volume of distribution of 25 L/kg. Dose proportionality was established based on AUC over a range of 3 mg/kg BW to 9 mg/kg BW. Biliary excretion of the unchanged drug is the major route of elimination.
MICROBIOLOGY SECTION
MICROBIOLOGY
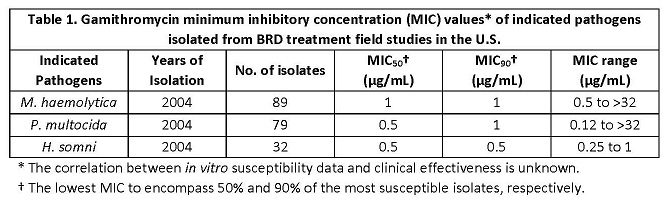
The minimum inhibitory concentrations (MIC's) of gamithromycin were determined for BRD isolates obtained from calves enrolled in BRD treatment field studies in the U.S. in 2004 using methods recommended by the Clinical and Laboratory Standards Institute (M31-A2). Isolates were obtained from pre-treatment nasopharyngeal swabs from each enrolled calf and from calves removed from the study due to BRD. The results are shown below in Table 1.
STORAGE AND HANDLING SECTION
STORAGE CONDITIONS
Store at or below 77°F (25°C) with excursions between 59-86°F (15-30°C). Use within 28 days of first puncture. If using multi-dosing equipment with a draw- off spike 4 gauge or smaller, puncture a maximum of 5 times. When using a needle larger than 16 gauge, or a draw-off spike larger than 4 gauge, discard any remaining product immediately after use.
HOW SUPPLIED SECTION
HOW SUPPLIED
GAMROZYNE is available in three ready-to-use bottle sizes. The 100, 250 and 500 mL bottles contain sufficient solution that will treat 10, 25 and 50 head of 550 lb (250 kg) cattle respectively.
