Mycophenolate Mofetil
These highlights do not include all the information needed to use MYCOPHENOLATE MOFETIL safely and effectively. See full prescribing information for MYCOPHENOLATE MOFETIL.MYCOPHENOLATE MOFETIL capsules, for oral useMYCOPHENOLATE MOFETIL tablets, for oral useInitial U.S. Approval: 1995
99b2eb58-8d14-48fc-bbe1-aacb711a783e
HUMAN PRESCRIPTION DRUG LABEL
Jul 24, 2025
Sandoz Inc
DUNS: 005387188
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Mycophenolate Mofetil
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Mycophenolate Mofetil
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 0781-5175-01
Mycophenolate Mofetil Tablets
500 mg*
PHARMACIST: Please dispense with Medication Guide provided separately.
Rx Only
100 Tablets
SANDOZ

Boxed Warning section
WARNING: EMBRYOFETAL TOXICITY, MALIGNANCIES, and SERIOUS INFECTIONS
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Mycophenolate mofetil (MMF) is indicated for the prophylaxis of organ rejection, in adult and pediatric recipients 3 months of age and older of allogeneic kidney [see Clinical Studies (14.1)], heart [see Clinical Studies (14.2)] or liver transplants [see Clinical Studies (14.3)], in combination with other immunosuppressants.
Mycophenolate mofetil is an antimetabolite immunosuppressant indicated for the prophylaxis of organ rejection in adult and pediatric recipients 3 months of age and older of allogeneic kidney, heart or liver transplants, in combination with other immunosuppressants. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Mycophenolate mofetil is contraindicated in patients with a history of hypersensitivity, including anaphylaxis, to mycophenolate mofetil (MMF), mycophenolic acid (MPA) or any component of the drug product [see Warnings and Precautions (5.8)].
•
History of hypersensitivity, including anaphylaxis, to mycophenolate mofetil, mycophenolic acid or any component of the drug product (4)
•
Patients allergic to Polysorbate 80 (present in mycophenolate mofetil IV) (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Embryofetal Toxicity
Use of MMF during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, kidney and nervous system. Females of reproductive potential must be made aware of these risks and must be counseled regarding pregnancy prevention and planning. Avoid use of MMF during pregnancy if safer treatment options are available [see Use in Specific Populations (8.1, 8.3)].
5.2 Lymphoma and Other Malignancies
Patients receiving immunosuppressants, including mycophenolate mofetil, are at increased risk of developing lymphomas and other malignancies, particularly of the skin [see Adverse Reactions (6.1)]. The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent. For patients with increased risk for skin cancer, exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen with a high protection factor.
Post-transplant lymphoproliferative disorder (PTLD) developed in 0.4% to 1% of patients receiving mycophenolate mofetil (2 g or 3 g) with other immunosuppressive agents in controlled clinical trials of kidney, heart and liver transplant patients [see Adverse Reactions (6.1)]. The majority of PTLD cases appear to be related to Epstein Barr Virus (EBV) infection. The risk of PTLD appears greatest in those individuals who are EBV seronegative, a population which includes many young children. In pediatric patients, no other malignancies besides PTLD were observed in clinical trials [see Adverse Reactions (6.1)].
5.3 Serious Infections
Patients receiving immunosuppressants, including mycophenolate mofetil, are at increased risk of developing bacterial, fungal, protozoal and new or reactivated viral infections, including opportunistic infections. The risk increases with the total immunosuppressive load. These infections may lead to serious outcomes, including hospitalizations and death [see Adverse Reactions (6.1, 6.2)].
Serious viral infections reported include:
•
Polyomavirus-associated nephropathy (PVAN), especially due to BK virus infection
•
JC virus-associated progressive multifocal leukoencephalopathy (PML), and
•
Cytomegalovirus (CMV) infections: CMV seronegative transplant patients who receive an organ from a CMV seropositive donor are at highest risk of CMV viremia and CMV disease.
•
Viral reactivation in patients infected with Hepatitis B and C
•
COVID-19
Consider dose reduction or discontinuation of mycophenolate mofetil in patients who develop new infections or reactivate viral infections, weighing the risk that reduced immunosuppression represents to the functioning allograft.
PVAN, especially due to BK virus infection, is associated with serious outcomes, including deteriorating renal function and renal graft loss [see Adverse Reactions (6.2)]. Patient monitoring may help detect patients at risk for PVAN.
PML, which is sometimes fatal, commonly presents with hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia [see Adverse Reactions (6.2)]. In immunosuppressed patients, physicians should consider PML in the differential diagnosis in patients reporting neurological symptoms.
The risk of CMV viremia and CMV disease is highest among transplant recipients seronegative for CMV at time of transplant who receive a graft from a CMV seropositive donor. Therapeutic approaches to limiting CMV disease exist and should be routinely provided. Patient monitoring may help detect patients at risk for CMV disease.
Viral reactivation has been reported in patients infected with HBV or HCV. Monitoring infected patients for clinical and laboratory signs of active HBV or HCV infection is recommended.
5.4 Blood Dyscrasias: Neutropenia and Pure Red Cell Aplasia (PRCA)
Severe neutropenia [absolute neutrophil count (ANC) <0.5 x 103/µL] developed in transplant patients receiving mycophenolate mofetil 3 g daily [see Adverse Reactions (6.1)]. Patients receiving mycophenolate mofetil should be monitored for neutropenia. Neutropenia has been observed most frequently in the period from 31 to 180 days post-transplant in patients treated for prevention of kidney, heart and liver rejection. The development of neutropenia may be related to mycophenolate mofetil itself, concomitant medications, viral infections, or a combination of these causes. If neutropenia develops (ANC <1.3 x 103/µL), dosing with mycophenolate mofetil should be interrupted or the dose reduced, appropriate diagnostic tests performed, and the patient managed appropriately [see Dosage and Administration (2.5)].
Patients receiving mycophenolate mofetil should be instructed to report immediately any evidence of infection, unexpected bruising, bleeding or any other manifestation of bone marrow depression.
Consider monitoring with complete blood counts weekly for the first month, twice monthly for the second and third months, and monthly for the remainder of the first year.
Cases of pure red cell aplasia (PRCA) have been reported in patients treated with mycophenolate mofetil in combination with other immunosuppressive agents. In some cases, PRCA was found to be reversible with dose reduction or cessation of mycophenolate mofetil therapy. In transplant patients, however, reduced immunosuppression may place the graft at risk.
5.5 Gastrointestinal Complications
Gastrointestinal bleeding requiring hospitalization, ulceration and perforations were observed in clinical trials. Physicians should be aware of these serious adverse effects particularly when administering mycophenolate mofetil to patients with a gastrointestinal disease.
5.6 Patients with Hypoxanthine-Guanine Phosphoribosyl-Transferase
Deficiency (HGPRT)
Mycophenolate mofetil is an inosine monophosphate dehydrogenase (IMPDH) inhibitor; therefore it should be avoided in patients with hereditary deficiencies of hypoxanthine-guanine phosphoribosyl-transferase (HGPRT) such as Lesch-Nyhan and Kelley-Seegmiller syndromes because it may cause an exacerbation of disease symptoms characterized by the overproduction and accumulation of uric acid leading to symptoms associated with gout such as acute arthritis, tophi, nephrolithiasis or urolithiasis and renal disease including renal failure.
5.7 Acute Inflammatory Syndrome Associated with Mycophenolate Products
Acute inflammatory syndrome (AIS) has been reported with the use of MMF and mycophenolate products, and some cases have resulted in hospitalization. AIS is a paradoxical pro-inflammatory reaction characterized by fever, arthralgias, arthritis, muscle pain and elevated inflammatory markers including, C-reactive protein and erythrocyte sedimentation rate, without evidence of infection or underlying disease recurrence. Symptoms occur within weeks to months of initiation of treatment or a dose increase. After discontinuation, improvement of symptoms and inflammatory markers are usually observed within 24 to 48 hours.
Monitor patients for symptoms and laboratory parameters of AIS when starting treatment with mycophenolate products or when increasing the dosage. Discontinue treatment and consider other treatment alternatives based on the risk and benefit for the patient.
5.8 Hypersensitivity Reactions
Postmarketing cases of hypersensitivity reactions, including angioedema and anaphylaxis, have been reported with mycophenolate mofetil. These reactions generally occurred within hours to the next day after initiating mycophenolate mofetil. If signs or symptoms of hypersensitivity reaction occur, discontinue mycophenolate mofetil; treat and monitor until symptoms resolve [see Contraindications (4)].
5.9 Immunizations
During treatment with mycophenolate mofetil, the use of live attenuated vaccines should be avoided (e.g., intranasal influenza, measles, mumps, rubella, oral polio, BCG, yellow fever, varicella, and TY21a typhoid vaccines) and patients should be advised that vaccinations may be less effective. Advise patients to discuss with the physician before seeking any immunizations.
5.12 Blood Donation
Patients should not donate blood during therapy and for at least 6 weeks following discontinuation of mycophenolate mofetil because their blood or blood products might be administered to a female of reproductive potential or a pregnant woman.
5.13 Semen Donation
Based on animal data, men should not donate semen during therapy and for 90 days following discontinuation of mycophenolate mofetil [see Use In Specific Populations (8.3)].
5.14 Effect of Concomitant Medications on Mycophenolic Acid Concentrations
A variety of drugs have potential to alter systemic MPA exposure when co- administered with mycophenolate mofetil. Therefore, determination of MPA concentrations in plasma before and after making any changes to immunosuppressive therapy, or when adding or discontinuing concomitant medications, may be appropriate to ensure MPA concentrations remain stable.
5.15 Potential Impairment of Ability to Drive or Operate Machinery
Mycophenolate mofetil may impact the ability to drive and use machines. Patients should avoid driving or using machines if they experience somnolence, confusion, dizziness, tremor, or hypotension during treatment with mycophenolate mofetil [see Adverse Reactions (6.1)].
•
Blood Dyscrasias (Neutropenia, Red Blood Cell Aplasia): Monitor with blood tests; consider treatment interruption or dose reduction. (5.4)
•
Gastrointestinal Complications: Monitor for complications such as bleeding, ulceration and perforations, particularly in patients with underlying gastrointestinal disorders. (5.5)
•
Hypoxanthine-Guanine Phosphoribosyl-Transferase Deficiency: Avoid use of mycophenolate mofetil. (5.6)
•
Acute Inflammatory Syndrome Associated with Mycophenolate Products: Monitor for this paradoxical inflammatory reaction. (5.7)
•
Hypersensitivity Reactions: Discontinue mycophenolate mofetil; treat and monitor until signs and symptoms resolve. (5.8)
•
Immunizations: Avoid live attenuated vaccines. (5.9)
•
Blood Donation: Avoid during therapy and for 6 weeks thereafter. (5.12)
•
Semen Donation: Avoid during therapy and for 90 days thereafter. (5.13)
•
Potential Impairment on Driving and Use of Machinery: Mycophenolate mofetil may affect ability to drive or operate machinery. (5.15)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
•
Embryofetal Toxicity [see Warnings and Precautions (5.1)]
•
Lymphomas and Other Malignancies [see Warnings and Precautions (5.2)]
•
Serious Infections [see Warnings and Precautions (5.3)]
•
Blood Dyscrasias: Neutropenia, Pure Red Cell Aplasia [see Warnings and Precautions (5.4)]
•
Gastrointestinal Complications [see Warnings and Precautions (5.5)]
•
Acute Inflammatory Syndrome Associated with Mycophenolate Products [see Warnings and Precautions (5.7)]
•
Hypersensitivity Reactions [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
An estimated total of 1,557 adult patients received mycophenolate mofetil during pivotal clinical trials in the prevention of acute organ rejection. Of these, 991 were included in the three renal studies, 277 were included in one hepatic study, and 289 were included in one cardiac study. Patients in all study arms also received cyclosporine and corticosteroids.
The data described below primarily derive from five randomized, active- controlled double-blind 12-month trials of mycophenolate mofetil in de novo kidney (3) heart (1) and liver (1) transplant patients [see Clinical Studies (14.1, 14.2, and 14.3)].
Mycophenolate mofetil Oral
The incidence of adverse reactions for mycophenolate mofetil was determined in five randomized, comparative, double-blind trials in the prevention of rejection in kidney, heart and liver transplant patients (two active- and one placebo-controlled trials, one active-controlled trial, and one active- controlled trial, respectively) [see Clinical Studies (14.1, 14.2 and 14.3)].
The three de novo kidney studies with 12-month duration compared two dose levels of oral mycophenolate mofetil (1 g twice daily and 1.5 g twice daily) with azathioprine (2 studies) or placebo (1 study) when administered in combination with cyclosporine (Sandimmune®) and corticosteroids to prevent acute rejection episodes. One study also included anti-thymocyte globulin (ATGAM®) induction therapy.
In the de novo heart transplantation study with 12-month duration, patients received mycophenolate mofetil 1.5 g twice daily (n=289) or azathioprine 1.5 to 3 mg/kg/day (n=289), in combination with cyclosporine (Sandimmune® or Neoral®) and corticosteroids as maintenance immunosuppressive therapy.
In the de novo liver transplantation study with 12-month duration, patients received mycophenolate mofetil 1 g twice daily intravenously for up to 14 days followed by mycophenolate mofetil 1.5 g twice daily orally or azathioprine 1 to 2 mg/kg/day intravenously followed by azathioprine 1 to 2 mg/kg/day orally, in combination with cyclosporine (Neoral®) and corticosteroids as maintenance immunosuppressive therapy. The total number of patients enrolled was 565.
Approximately 53% of the kidney transplant patients, 65% of the heart transplant patients, and 48% of the liver transplant patients were treated for more than 1 year. Adverse reactions reported in ≥20% of patients in the mycophenolate mofetil treatment groups are presented below. The safety data of three kidney transplantation studies are pooled together.
**Table 5. Adverse Reactions in Controlled Studies of De Novo Kidney, Heart or Liver Transplantation Reported in ≥20% of Patients in the Mycophenolate Mofetil Group**
|
Adverse Drug Reaction System Organ Class |
Kidney Studies |
Heart Study |
Liver Study | ||||
2 g/day (n=501) or 3g/day (n=490) |
AZA 1 to 2 mg/kg/day or |
Placebo |
3 g/day |
AZA day |
3 g/day |
AZA day | |
|
(n=991) |
(n=326) |
(n=166) |
(n=289) |
(n=289) |
(n=277) |
(n=287) | |
|
% |
% |
% |
% |
% |
% |
% | |
|
Infections and infestations | |||||||
|
Bacterial infections |
39.9 |
33.7 |
37.3 |
|
|
27.4 |
26.5 |
|
Viral infections |
–* |
– |
– |
31.1 |
24.9 |
|
|
|
Blood and lymphatic system disorders | |||||||
|
Anemia |
20 |
23.6 |
2.4 |
45 |
47.1 |
43 |
53 |
|
Ecchymosis |
|
|
|
20.1 |
9.7 |
|
|
|
Leukocytosis |
– |
– |
– |
42.6 |
37.4 |
22.4 |
21.3 |
|
Leukopenia |
28.6 |
24.8 |
4.2 |
34.3 |
43.3 |
45.8 |
39 |
|
Thrombocytopenia |
|
|
|
24.2 |
28 |
38.3 |
42.2 |
|
Metabolism and nutrition disorders | |||||||
|
Hypercholesterolemia |
|
|
|
46 |
43.9 |
– |
– |
|
Hyperglycemia |
|
|
|
48.4 |
53.3 |
43.7 |
48.8 |
|
Hyperkalemia |
|
|
|
|
|
22 |
23.7 |
|
Hypocalcemia |
|
|
|
|
|
30 |
30 |
|
Hypokalemia |
|
|
|
32.5 |
26.3 |
37.2 |
41.1 |
|
Hypomagnesemia |
|
|
|
20.1 |
14.2 |
39 |
37.6 |
|
Psychiatric disorders | |||||||
|
Depression |
|
|
|
20.1 |
15.2 |
|
|
|
Insomnia |
– |
– |
– |
43.3 |
39.8 |
52.3 |
47 |
|
Nervous system disorders | |||||||
|
Dizziness |
|
|
|
34.3 |
33.9 |
|
|
|
Headache |
– |
– |
– |
58.5 |
55.4 |
53.8 |
49.1 |
|
Tremor |
– |
– |
– |
26.3 |
25.6 |
33.9 |
35.5 |
|
Cardiac disorders | |||||||
|
Tachycardia |
– |
– |
– |
22.8 |
21.8 |
22 |
15.7 |
|
Vascular disorders | |||||||
|
Hypertension |
27.5 |
32.2 |
19.3 |
78.9 |
74 |
62.1 |
59.6 |
|
Hypotension |
|
|
|
34.3 |
40.1 |
|
|
|
Respiratory, thoracic and mediastinal disorders | |||||||
|
Cough |
|
|
|
40.5 |
32.2 |
|
|
|
Dyspnea |
|
|
|
44.3 |
44.3 |
31 |
30.3 |
|
Pleural effusion |
|
|
|
|
|
34.3 |
35.9 |
|
Gastrointestinal disorders | |||||||
|
Abdominal pain |
22.4 |
23 |
11.4 |
41.9 |
39.4 |
62.5 |
51.2 |
|
Constipation |
– |
– |
– |
43.6 |
38.8 |
37.9 |
38.3 |
|
Decreased appetite |
|
|
|
|
|
25.3 |
17.1 |
|
Diarrhea |
30.4 |
20.9 |
13.9 |
52.6 |
39.4 |
51.3 |
49.8 |
|
Dyspepsia |
|
|
|
22.1 |
22.1 |
22.4 |
20.9 |
|
Nausea |
|
|
|
56.1 |
60.2 |
54.5 |
51.2 |
|
Vomiting |
|
|
|
39.1 |
34.6 |
32.9 |
33.4 |
|
Hepatobiliary disorders | |||||||
|
Blood lactate dehydrogenase increased |
– |
– |
– |
23.5 |
18.3 |
– |
– |
|
Hepatic enzyme increased |
|
|
|
|
|
24.9 |
19.2 |
|
Skin and subcutaneous tissues disorders | |||||||
|
Rash |
– |
– |
– |
26 |
20.8 |
|
|
|
Renal and urinary disorders | |||||||
|
Blood creatinine increased |
– |
– |
– |
42.2 |
39.8 |
– |
– |
|
Blood urea increased |
– |
– |
– |
36.7 |
34.3 |
– |
– |
|
General disorders and administration site conditions | |||||||
|
Asthenia |
|
|
|
49.1 |
41.2 |
35.4 |
33.8 |
|
Edema† |
21 |
28.2 |
8.4 |
67.5 |
55.7 |
48.4 |
47.7 |
|
Pain‡ |
24.8 |
32.2 |
9.6 |
79.2 |
77.5 |
74 |
77.5 |
|
Pyrexia |
|
|
|
56.4 |
53.6 |
52.3 |
56.1 |
- “-“Indicates that the incidence was below the cutoff value of 20% for inclusion in the table.
† “Edema” includes peripheral edema, facial edema, scrotal edema.
‡ “Pain” includes musculoskeletal pain (myalgia, neck pain, back pain).
In the three de novo kidney studies, patients receiving 2 g/day of mycophenolate mofetil had an overall better safety profile than did patients receiving 3 g/day of mycophenolate mofetil.
Post-transplant lymphoproliferative disease (PTLD, pseudolymphoma) developed in 0.4% to 1% of patients receiving mycophenolate mofetil (2 g or 3 g daily) with other immunosuppressive agents in controlled clinical trials of kidney, heart and liver transplant patients followed for at least 1 year [see Warnings and Precautions (5.2)]. Non-melanoma skin carcinomas occurred in 1.6% to 4.2% of patients, other types of malignancy in 0.7% to 2.1% of patients. Three-year safety data in kidney and heart transplant patients did not reveal any unexpected changes in incidence of malignancy compared to the 1-year data. In pediatric patients, PTLD was observed in 1.35% (2/148) by 12 months post- transplant.
Cytopenias, including leukopenia, anemia, thrombocytopenia and pancytopenia are a known risk associated with mycophenolate and may lead or contribute to the occurrence of infections and hemorrhages [see Warnings and Precautions (5.3)]. Severe neutropenia (ANC <0.5 x 103/µL) developed in up to 2% of kidney transplant patients, up to 2.8% of heart transplant patients and up to 3.6% of liver transplant patients receiving mycophenolate mofetil 3 g daily [see Warnings and Precautions (5.4) and Dosage and Administration (2.5)].
The most common opportunistic infections in patients receiving mycophenolate mofetil with other immunosuppressants were mucocutaneous candida, CMV viremia/syndrome, and herpes simplex. The proportion of patients with CMV viremia/syndrome was 13.5%. In patients receiving mycophenolate mofetil (2 g or 3 g) in controlled studies for prevention of kidney, heart or liver rejection, fatal infection/sepsis occurred in approximately 2% of kidney and heart patients and in 5% of liver patients [see Warnings and Precautions (5.3)].
The most serious gastrointestinal disorders reported were ulceration and hemorrhage, which are known risks associated with mycophenolate mofetil. Mouth, esophageal, gastric, duodenal, and intestinal ulcers often complicated by hemorrhage, as well as hematemesis, melena, and hemorrhagic forms of gastritis and colitis were commonly reported during the pivotal clinical trials, while the most common gastrointestinal disorders were diarrhea, nausea and vomiting. Endoscopic investigation of patients with mycophenolate mofetil -related diarrhea revealed isolated cases of intestinal villous atrophy [see Warnings and Precautions (5.5)].
The following adverse reactions were reported with 3% to <20% incidence in kidney, heart, and liver transplant patients treated with mycophenolate mofetil, in combination with cyclosporine and corticosteroids.
**Table 6. Adverse Reactions in Controlled Studies of De Novo Kidney, Heart or Liver Transplantation Reported in 3% to <20% of Patients Treated with Mycophenolate Mofetil in Combination with Cyclosporine and Corticosteroids**
|
System Organ Class |
Adverse Reactions |
|
Body as a Whole |
cellulitis, chills, hernia, malaise |
|
Infections and Infestations |
fungal infections |
|
Hematologic and Lymphatic |
coagulation disorder, ecchymosis, pancytopenia |
|
Urogenital |
hematuria |
|
Cardiovascular |
hypotension |
|
Metabolic and Nutritional |
acidosis, alkaline phosphatase increased, hyperlipemia, hypophosphatemia, weight loss |
|
Digestive |
esophagitis, flatulence, gastritis, gastrointestinal hemorrhage, hepatitis, ileus, nausea and vomiting, stomach ulcer, stomatitis |
|
Neoplasm benign, malignant and unspecified |
neoplasm |
|
Skin and Appendages |
skin benign neoplasm, skin carcinoma |
|
Psychiatric |
confusional state |
|
Nervous |
hypertonia, paresthesia, somnolence |
|
Musculoskeletal |
arthralgia, myasthenia |
Pediatrics
The type and frequency of adverse events in a clinical study for prevention of kidney allograft rejection in 100 pediatric patients 3 months to 18 years of age dosed with mycophenolate mofetil oral suspension 600 mg/m2 twice daily (up to 1 g twice daily) were generally similar to those observed in adult patients dosed with mycophenolate mofetil capsules at a dose of 1 g twice daily with the exception of abdominal pain, fever, infection, pain, sepsis, diarrhea, vomiting, pharyngitis, respiratory tract infection, hypertension, leukopenia, and anemia, which were observed in a higher proportion in pediatric patients.
Safety information in pediatric heart transplant or pediatric liver transplant patients treated with mycophenolate mofetil is supported by an open-label study in pediatric liver transplant patients and publications; the type and frequency of the reported adverse reactions are consistent with those observed in pediatric patients following renal transplant and in adults.
Geriatrics
Geriatric patients (≥65 years), particularly those who are receiving mycophenolate mofetil as part of a combination immunosuppressive regimen, may be at increased risk of certain infections (including cytomegalovirus [CMV] tissue invasive disease) and possibly gastrointestinal hemorrhage and pulmonary edema, compared to younger individuals [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of mycophenolate mofetil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
•
Embryo-Fetal Toxicity: Congenital malformations and spontaneous abortions, mainly in the first trimester, have been reported following exposure to mycophenolate mofetil (MMF) in combination with other immunosuppressants during pregnancy [see Warnings and Precautions (5.1), and Use in Specific Populations (8.1), (8.3)]. Congenital malformations include:
-Facial malformations: cleft lip, cleft palate, micrognathia, hypertelorism of the orbits
-Abnormalities of the ear and eye: abnormally formed or absent external/middle ear, coloboma, microphthalmos
-Malformations of the fingers: polydactyly, syndactyly, brachydactyly
-Cardiac abnormalities: atrial and ventricular septal defects
-Esophageal malformations: esophageal atresia
-Nervous system malformations: such as spina bifida.
•
Digestive: Colitis, pancreatitis
•
Hematologic and Lymphatic: Bone marrow failure, cases of pure red cell aplasia (PRCA) and hypogammaglobulinemia have been reported in patients treated with mycophenolate mofetil in combination with other immunosuppressive agents [see Warnings and Precautions (5.4)].
•
Immune: Hypersensitivity reactions, including anaphylaxis and angioedema [see Warnings and Precautions (5.8)], hypogammaglobinemia.
•
Infections: Meningitis, infectious endocarditis, tuberculosis, atypical mycobacterial infection, progressive multifocal leukoencephalopathy, BK virus infection, viral reactivation of hepatitis B and hepatitis C, protozoal infections [see Warnings and Precautions (5.3)].
•
Respiratory: Bronchiectasis, interstitial lung disease, fatal pulmonary fibrosis, have been reported rarely and should be considered in the differential diagnosis of pulmonary symptoms ranging from dyspnea to respiratory failure in post-transplant patients receiving mycophenolate mofetil.
•
Vascular: Lymphocele.
The most common adverse reactions in clinical trials (20 % or greater) include diarrhea, leukopenia, infection, vomiting, and there is evidence of a higher frequency of certain types of infections e.g., opportunistic infection. (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc., at 1-800-525-8747 or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.com.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mycophenolate mofetil (MMF) is absorbed following oral administration and hydrolyzed to mycophenolic acid (MPA), the active metabolite. MPA is a selective uncompetitive inhibitor of the two isoforms (type I and type II) of inosine monophosphate dehydrogenase (IMPDH) leading to inhibition of the de novo pathway of guanosine nucleotide synthesis and blocks DNA synthesis. The mechanism of action of MPA is multifaceted and includes effects on cellular checkpoints responsible for metabolic programming of lymphocytes. MPA shifts transcriptional activities in lymphocytes from a proliferative state to catabolic processes. In vitro studies suggest that MPA modulates transcriptional activities in human CD4+ T-lymphocytes by suppressing the Akt/mTOR and STAT5 pathways that are relevant to metabolism and survival, leading to an anergic state of T-cells whereby the cells become less responsive to antigenic stimulation. Additionally, MPA enhanced the expression of negative co-stimulators such as CD70, PD-1, CTLA-4, and transcription factor FoxP3 as well as decreased the expression of positive co-stimulators CD27 and CD28.
MPA decreases proliferative responses of T- and B-lymphocytes to both mitogenic and allo-antigenic stimulation, antibody responses, as well as the production of cytokines from lymphocytes and monocytes such as GM-CSF, IFN-Ɣ, IL-17, and TNF-α. Additionally, MPA prevents the glycosylation of lymphocyte and monocyte glycoproteins that are involved in intercellular adhesion to endothelial cells and may inhibit recruitment of leukocytes into sites of inflammation and graft rejection.
Overall, the effect of MPA is cytostatic and reversible.
12.2 Pharmacodynamics
There is a lack of information regarding the pharmacodynamic effects of MMF.
12.3 Pharmacokinetics
Absorption
Following oral and intravenous administration, MMF undergoes complete conversion to MPA, the active metabolite. In 12 healthy volunteers, the mean absolute bioavailability of oral MMF relative to intravenous MMF was 94%. Two 500 mg mycophenolate mofetil tablets have been shown to be bioequivalent to four 250 mg mycophenolate mofetil capsules. Five mL of the 200 mg/mL constituted mycophenolate mofetil oral suspension have been shown to be bioequivalent to four 250 mg capsules.
The mean (±SD) pharmacokinetic parameters estimates for MPA following the administration of MMF given as single doses to healthy volunteers, and multiple doses to kidney, heart, and liver transplant patients, are shown in Table. The area under the plasma-concentration time curve (AUC) for MPA appears to increase in a dose-proportional fashion in kidney transplant patients receiving multiple oral doses of MMF up to a daily dose of 3 g (1.5g twice daily) (seeTable 10).
**Table 10. Pharmacokinetic Parameters for MPA [mean (±SD)] Following Administration of MMF to Healthy Volunteers (Single Dose), and Kidney, Heart, and Liver Transplant Patients (Multiple Doses)**
|
Healthy Volunteers |
Dose/Route |
T****max |
C****max |
Total AUC (mcg∙h/mL) |
|
Single dose |
1 g/oral |
0.80 |
24.5 |
63.9 |
|
Kidney Transplant Patients (twice daily dosing) Time After Transplantation |
Dose/Route |
T****max |
C****max |
Interdosing Interval AUC (0–12h) (mcg∙h/mL) |
|
5 days |
1 g/iv |
1.58 |
12 |
40.8 |
|
6 days |
1 g/oral |
1.33 |
10.7 |
32.9 |
|
Early (Less than 40 days) |
1 g/oral |
1.31 |
8.16 |
27.3 |
|
Early (Less than 40 days) |
1.5 g/oral |
1.21 |
13.5 |
38.4 |
|
Late (Greater than 3 months) |
1.5 g/oral |
0.90 |
24.1 |
65.3 |
|
Heart transplant Patients (twice daily dosing) Time After Transplantation |
Dose/Route |
T****max |
C****max |
Interdosing Interval AUC (0–12h) (mcg∙h/mL) |
|
Early (Day before discharge) |
1.5 g/oral |
1.8 |
11.5 |
43.3 |
|
Late (Greater than 6 months) |
1.5 g/oral |
1.1 |
20 |
54.1* (±20.4) |
|
Liver transplant Patients (twice daily dosing) Time After Transplantation |
Dose/Route |
T****max |
C****max |
Interdosing Interval AUC (0–12h) (mcg∙h/mL) |
|
4 to 9 days |
1 g/iv |
1.50 |
17 |
34 |
|
Early (5 to 8 days) |
1.5 g/oral |
1.15 |
13.1 |
29.2 |
|
Late (Greater than 6 months) |
1.5 g/oral |
1.54 |
19.3 |
49.3 |
*AUC(0-12h) values quoted are extrapolated from data from samples collected over 4 hours.
In the early post-transplant period (less than 40 days post-transplant), kidney, heart, and liver transplant patients had mean MPA AUCs approximately 20% to 41% lower and mean Cmax approximately 32% to 44% lower compared to the late transplant period (i.e., 3 to 6 months post-transplant) (non-stationarity in MPA pharmacokinetics).
Mean MPA AUC values following administration of 1 g twice daily intravenous mycophenolate mofetil over 2 hours to kidney transplant patients for 5 days were about 24% higher than those observed after oral administration of a similar dose in the immediate post-transplant phase.
In liver transplant patients, administration of 1 g twice daily intravenous mycophenolate mofetil followed by 1.5 g twice daily oral mycophenolate mofetil resulted in mean MPA AUC estimates similar to those found in kidney transplant patients administered 1 g mycophenolate mofetil twice daily.
Effect of Food
Food (27 g fat, 650 calories) had no effect on the extent of absorption (MPA AUC) of MMF when administered at doses of 1.5 g twice daily to kidney transplant patients. However, MPA Cmax was decreased by 40% in the presence of food [see Dosage and Administration (2.1)].
Distribution
The mean (±SD) apparent volume of distribution of MPA in 12 healthy volunteers was approximately 3.6 (±1.5) L/kg. At clinically relevant concentrations, MPA is 97% bound to plasma albumin. The phenolic glucuronide metabolite of MPA (MPAG) is 82% bound to plasma albumin at MPAG concentration ranges that are normally seen in stable kidney transplant patients; however, at higher MPAG concentrations (observed in patients with kidney impairment or delayed kidney graft function), the binding of MPA may be reduced as a result of competition between MPAG and MPA for protein binding. Mean blood to plasma ratio of radioactivity concentrations was approximately 0.6 indicating that MPA and MPAG do not extensively distribute into the cellular fractions of blood.
In vitro studies to evaluate the effect of other agents on the binding of MPA to human serum albumin (HSA) or plasma proteins showed that salicylate (at 25 mg/dL with human serum albumin) and MPAG (at ≥ 460 mcg/mL with plasma proteins) increased the free fraction of MPA. MPA at concentrations as high as 100 mcg/mL had little effect on the binding of warfarin, digoxin or propranolol, but decreased the binding of theophylline from 53% to 45% and phenytoin from 90% to 87%.
Elimination
Mean (±SD) apparent half-life and plasma clearance of MPA are 17.9 (±6.5) hours and 193 (±48) mL/min following oral administration and 16.6 (±5.8) hours and 177 (±31) mL/min following intravenous administration, respectively.
Metabolism
The parent drug, MMF, can be measured systemically during the intravenous infusion; however, approximately 5 minutes after the infusion is stopped or after oral administration, MMF concentrations are below the limit of quantitation (0.4 mcg/mL).
Metabolism to MPA occurs pre-systemically after oral dosing. MPA is metabolized principally by glucuronyl transferase to form MPAG, which is not pharmacologically active. In vivo, MPAG is converted to MPA during enterohepatic recirculation. The following metabolites of the 2-hydroxyethyl- morpholino moiety are also recovered in the urine following oral administration of MMF to healthy subjects: N-(2-carboxymethyl)-morpholine, N-(2-hydroxyethyl)-morpholine, and the N-oxide of N-(2-hydroxyethyl)-morpholine.
Due to the enterohepatic recirculation of MPAG/MPA, secondary peaks in the plasma MPA concentration-time profile are usually observed 6 to 12 hours post- dose. Bile sequestrants, such as cholestyramine, reduce MPA AUC by interfering with this enterohepatic recirculation of the drug [see Overdosage (10) and Drug Interaction Studies below].
Excretion
Negligible amount of drug is excreted as MPA (less than 1% of dose) in the urine. Orally administered radiolabeled MMF resulted in complete recovery of the administered dose, with 93% of the administered dose recovered in the urine and 6% recovered in feces. Most (about 87%) of the administered dose is excreted in the urine as MPAG. At clinically encountered concentrations, MPA and MPAG are usually not removed by hemodialysis. However, at high MPAG plasma concentrations (>100 mcg/mL), small amounts of MPAG are removed.
Increased plasma concentrations of MMF metabolites (MPA 50% increase and MPAG about a 3-fold to 6-fold increase) are observed in patients with renal insufficiency [see Specific Populations].
Specific Populations
Patients with Renal Impairment
The mean (±SD) pharmacokinetic parameters for MPA following the administration of oral MMF given as single doses to non-transplant subjects with renal impairment are presented inTable 11.
In a single-dose study, MMF was administered as a capsule or as an intravenous infusion over 40 minutes. Plasma MPA AUC observed after oral dosing to volunteers with severe chronic renal impairment (GFR < 25 mL/min/1.73 m2) was about 75% higher relative to that observed in healthy volunteers (GFR > 80 mL/min/1.73 m2). In addition, the single-dose plasma MPAG AUC was 3-fold to 6-fold higher in volunteers with severe renal impairment than in volunteers with mild renal impairment or healthy volunteers, consistent with the known renal elimination of MPAG. No data are available on the safety of long-term exposure to this level of MPAG.
Plasma MPA AUC observed after single-dose (1 g) intravenous dosing to volunteers (n=4) with severe chronic renal impairment (GFR < 25 mL/min/1.73 m2) was 62.4 mcg•h/mL (±19.3). Multiple dosing of MMF in patients with severe chronic renal impairment has not been studied.
Patients with Delayed Graft Function or Nonfunction
In patients with delayed renal graft function post-transplant, mean MPA AUC(0-12h) was comparable to that seen in post-transplant patients without delayed renal graft function. There is a potential for a transient increase in the free fraction and concentration of plasma MPA in patients with delayed renal graft function. However, dose adjustment does not appear to be necessary in patients with delayed renal graft function. Mean plasma MPAG AUC(0-12h) was 2-fold to 3-fold higher than in post-transplant patients without delayed renal graft function [see Dosage and Administration (2.5)].
In eight patients with primary graft non-function following kidney transplantation, plasma concentrations of MPAG accumulated about 6-fold to 8-fold after multiple dosing for 28 days. Accumulation of MPA was about 1-fold to 2-fold.
The pharmacokinetics of MMF are not altered by hemodialysis. Hemodialysis usually does not remove MPA or MPAG. At high concentrations of MPAG (>100 mcg/mL), hemodialysis removes only small amounts of MPAG.
Patients with Hepatic Impairment
The mean (± SD) pharmacokinetic parameters for MPA following the administration of oral MMF given as single doses to non-transplant subjects with hepatic impairment is presented inTable 11.
In a single-dose (1 g oral) study of 18 volunteers with alcoholic cirrhosis and 6 healthy volunteers, hepatic MPA glucuronidation processes appeared to be relatively unaffected by hepatic parenchymal disease when pharmacokinetic parameters of healthy volunteers and alcoholic cirrhosis patients within this study were compared. However, it should be noted that for unexplained reasons, the healthy volunteers in this study had about a 50% lower AUC as compared to healthy volunteers in other studies, thus making comparisons between volunteers with alcoholic cirrhosis and healthy volunteers difficult. In a single-dose (1 g intravenous) study of 6 volunteers with severe hepatic impairment (aminopyrine breath test less than 0.2% of dose) due to alcoholic cirrhosis, MMF was rapidly converted to MPA. MPA AUC was 44.1 mcg•h/mL (±15.5).
**Table 11. Pharmacokinetic Parameters for MPA [mean (±SD)] Following Single Doses of MMF Capsules in Chronic Renal and Hepatic Impairment**
|
Pharmacokinetic Parameters for Renal Impairment | ||||
|
Dose |
T****max |
C****max |
AUC(0–96h) | |
|
Healthy Volunteers |
1 g |
0.75 |
25.3 |
45 |
|
Mild Renal Impairment |
1 g |
0.75 |
26 |
59.9 |
|
Moderate Renal Impairment |
1 g |
0.75 |
19 |
52.9 |
|
Severe Renal Impairment |
1 g |
1 |
16.3 |
78.6 |
|
Pharmacokinetic Parameters for Hepatic Impairment | ||||
|
Dose |
T****max |
C****max |
AUC(0–48h) | |
|
Healthy Volunteers |
1 g |
0.63 |
24.3 |
29 |
|
Alcoholic Cirrhosis |
1 g |
0.85 |
22.4 |
29.8 |
Pediatric Patients
The pharmacokinetic parameters of MPA and MPAG have been evaluated in 55 pediatric patients (ranging from 1 year to 18 years of age) receiving mycophenolate mofetil oral suspension at a dose of 600 mg/m2 twice daily (up to a maximum of 1 g twice daily) after allogeneic kidney transplantation. The pharmacokinetic data for MPA is provided inTable 12.
**Table 12. Mean (±SD) Computed Pharmacokinetic Parameters for MPA by Age and Time after Allogeneic Kidney Transplantation**
|
Age Group (n) |
Time |
T****max |
Dose Adjusted******* C****max** |
Dose Adjusted***** |
|---|---|---|---|---|
|
1 to less than 2 yr (6)§ |
Early (Day 7) |
3.03 (4.70) |
10.3 (5.80) |
22.5 (6.66) |
|
1 to less than 6 yr (17) |
1.63 (2.85) |
13.2 (7.16) |
27.4 (9.54) | |
|
6 to less than 12 yr (16) |
|
13.1 (6.30) |
33.2 (12.1) | |
|
12 to 18 yr (21) |
1.16 (0.830) |
11.7 (10.7) |
26.3 (9.14) † | |
|
1 to less than 2 yr (4)§ |
Late (Month 3) |
0.725 (0.276) |
23.8 (13.4) |
47.4 (14.7) |
|
1 to less than 6 yr (15) |
0.989 (0.511) |
22.7 (10.1) |
49.7 (18.2) | |
|
6 to less than 12 yr (14) |
1.21 (0.532) |
27.8 (14.3) |
61.9 (19.6) | |
|
12 to 18 yr (17) |
0.978 (0.484) |
17.9 (9.57) |
| |
|
1 to less than 2 yr (4)§ |
Late (Month 9) |
0.604 (0.208) |
25.6 (4.25) |
55.8 (11.6) |
|
1 to less than 6 yr (12) |
0.869 (0.479) |
30.4 (9.16) |
61 (10.7) | |
|
6 to less than 12 yr (11) |
1.12 (0.462) |
29.2 (12.6) |
66.8 (21.2) | |
|
12 to 18 yr (14) |
1.09 (0.518) |
18.1 (7.29) |
|
*adjusted to a dose of 600 mg/m2
†n=20
‡n=16
§a subset of 1 to <6 yr
The mycophenolate mofetil oral suspension dose of 600 mg/m2 twice daily (up to a maximum of 1 g twice daily) achieved mean MPA AUC values in pediatric patients similar to those seen in adult kidney transplant patients receiving mycophenolate mofetil capsules at a dose of 1 g twice daily in the early post- transplant period. There was wide variability in the data. As observed in adults, early post-transplant MPA AUC values were approximately 45% to 53% lower than those observed in the later post-transplant period (>3 months). MPA AUC values were similar in the early and late post-transplant period across the 1 to 18-year age range.
A comparison of dose-normalized (to 600 mg/m2) MPA AUC values in 12 pediatric kidney transplant patients less than 6 years of age at 9 months post- transplant with those values in 7 pediatric liver transplant patients [median age 17 months (range: 10 – 60 months)] and at 6 months and beyond post- transplant revealed that, at the same dose, there were on average 23% lower AUC values in the pediatric liver compared to pediatric kidney patients. This is consistent with the need for higher dosing in adult liver transplant patients compared to kidney transplant patients to achieve the same exposure.
In adult transplant patients administered the same dosage of mycophenolate mofetil, there is similar MPA exposure among kidney transplant and heart transplant patients. Based on the established similarity in MPA exposure between pediatric kidney transplant and adult kidney transplant patients at their respective approved doses, it is expected that MPA exposure at the recommended dosage will be similar in pediatric heart transplant and adult heart transplant patients.
Male and Female Patients
Data obtained from several studies were pooled to look at any gender-related differences in the pharmacokinetics of MPA (data were adjusted to 1 g oral dose). Mean (±SD) MPA AUC (0-12h) for males (n=79) was 32.0 (±14.5) and for females (n=41) was 36.5 (±18.8) mcg•h/mL while mean (±SD) MPA Cmax was 9.96 (±6.19) in the males and 10.6 (±5.64) mcg/mL in the females. These differences are not of clinical significance.
Geriatric Patients
The pharmacokinetics of mycophenolate mofetil and its metabolites have not been found to be altered in geriatric transplant patients when compared to younger transplant patients.
Drug Interaction Studies
Acyclovir
Coadministration of MMF (1 g) and acyclovir (800 mg) to 12 healthy volunteers resulted in no significant change in MPA AUC and Cmax. However, MPAG and acyclovir plasma AUCs were increased 10.6% and 21.9%, respectively.
Antacids with Magnesium and Aluminum Hydroxides
Absorption of a single dose of MMF (2 g) was decreased when administered to 10 rheumatoid arthritis patients also taking Maalox® TC (10 mL qid). The Cmax and AUC(0-24h) for MPA were 33% and 17% lower, respectively, than when MMF was administered alone under fasting conditions.
Proton Pump Inhibitors (PPIs)
Coadministration of PPIs (e.g., lansoprazole, pantoprazole) in single doses to healthy volunteers and multiple doses to transplant patients receiving mycophenolate mofetil has been reported to reduce the exposure to MPA. An approximate reduction of 30 to 70% in the Cmax and 25% to 35% in the AUC of MPA has been observed, possibly due to a decrease in MPA solubility at an increased gastric pH.
Cholestyramine
Following single-dose administration of 1.5 g MMF to 12 healthy volunteers pretreated with 4 g three times a day of cholestyramine for 4 days, MPA AUC decreased approximately 40%. This decrease is consistent with interruption of enterohepatic recirculation which may be due to binding of recirculating MPAG with cholestyramine in the intestine.
Cyclosporine
Cyclosporine (Sandimmune®) pharmacokinetics (at doses of 275 to 415 mg/day) were unaffected by single and multiple doses of 1.5 g twice daily of MMF in 10 stable kidney transplant patients. The mean (±SD) AUC (0-12h) and Cmax of cyclosporine after 14 days of multiple doses of MMF were 3290 (±822) ng•h/mL and 753 (±161) ng/mL, respectively, compared to 3245 (±1088) ng•h/mL and 700 (±246) ng/mL, respectively, 1 week before administration of MMF.
Cyclosporine A interferes with MPA enterohepatic recirculation. In kidney transplant patients, mean MPA exposure (AUC(0-12h)) was approximately 30 to 50% greater when MMF was administered without cyclosporine compared with when MMF was coadministered with cyclosporine. This interaction is due to cyclosporine inhibition of multidrug-resistance-associated protein 2 (MRP-2) transporter in the biliary tract, thereby preventing the excretion of MPAG into the bile that would lead to enterohepatic recirculation of MPA. This information should be taken into consideration when MMF is used without cyclosporine.
Drugs Affecting Glucuronidation
Concomitant administration of drugs inhibiting glucuronidation of MPA may increase MPA exposure (e.g., increase of MPA AUC(0-∞) by 35% was observed with concomitant administration of isavuconazole).
Concomitant administration of telmisartan and mycophenolate mofetil resulted in an approximately 30% decrease in MPA concentrations. Telmisartan changes MPA’s elimination by enhancing PPAR gamma (peroxisome proliferator-activated receptor gamma) expression, which in turn results in an enhanced UGT1A9 expression and glucuronidation activity.
Ganciclovir
Following single-dose administration to 12 stable kidney transplant patients, no pharmacokinetic interaction was observed between MMF (1.5 g) and intravenous ganciclovir (5 mg/kg). Mean (±SD) ganciclovir AUC and Cmax (n=10) were 54.3 (±19.0) mcg•h/mL and 11.5 (±1.8) mcg/mL, respectively, after coadministration of the two drugs, compared to 51 (±17) mcg•h/mL and 10.6 (±2) mcg/mL, respectively, after administration of intravenous ganciclovir alone. The mean (±SD) AUC and Cmax of MPA (n=12) after coadministration were 80.9 (±21.6) mcg•h/mL and 27.8 (±13.9) mcg/mL, respectively, compared to values of 80.3 (±16.4) mcg•h/mL and 30.9 (±11.2) mcg/mL, respectively, after administration of MMF alone.
Oral Contraceptives
A study of coadministration of mycophenolate mofetil (1 g twice daily) and combined oral contraceptives containing ethinylestradiol (0.02 mg to 0.04 mg) and levonorgestrel (0.05 mg to 0.20 mg), desogestrel (0.15 mg) or gestodene (0.05 mg to 0.10 mg) was conducted in 18 women with psoriasis over 3 consecutive menstrual cycles. Mean serum levels of LH, FSH and progesterone were not significantly affected. Mean AUC (0-24h) was similar for ethinylestradiol and 3-keto desogestrel; however, mean levonorgestrel AUC(0-24h) significantly decreased by about 15%. There was large inter-patient variability (%CV in the range of 60% to 70%) in the data, especially for ethinylestradiol.
Sevelamer
Concomitant administration of sevelamer and MMF in adult and pediatric patients decreased the mean MPA Cmax and AUC (0-12h) by 36% and 26% respectively.
Antimicrobials
Antimicrobials eliminating beta-glucuronidase-producing bacteria in the intestine (e.g. aminoglycoside, cephalosporin, fluoroquinolone, and penicillin classes of antimicrobials) may interfere with the MPAG/MPA enterohepatic recirculation thus leading to reduced systemic MPA exposure. Information concerning antibiotics is as follows:
•
Trimethoprim/Sulfamethoxazole: Following single-dose administration of MMF (1.5 g) to 12 healthy male volunteers on day 8 of a 10-day course of trimethoprim 160 mg/sulfamethoxazole 800 mg administered twice daily, no effect on the bioavailability of MPA was observed. The mean (±SD) AUC and Cmax of MPA after concomitant administration were 75.2 (±19.8) mcg•h/mL and 34.0 (±6.6) mcg/mL, respectively, compared to 79.2 (±27.9) mcg•h/mL and 34.2 (±10.7) mcg/mL, respectively, after administration of MMF alone.
•
Norfloxacin and Metronidazole: Following single-dose administration of MMF (1 g) to 11 healthy volunteers on day 4 of a 5-day course of a combination of norfloxacin and metronidazole, the mean MPA AUC(0-48h) was significantly reduced by 33% compared to the administration of MMF alone (p<0.05). The mean (±SD) MPA AUC(0-48h) after coadministration of MMF with norfloxacin or metronidazole separately was 48.3 (±24) mcg·h/mL and 42.7 (±23) mcg·h/mL, respectively, compared with 56.2 (±24) mcg·h/mL after administration of MMF alone.
•
Ciprofloxacin and Amoxicillin Plus Clavulanic Acid: A total of 64 mycophenolate mofetil -treated kidney transplant recipients received either oral ciprofloxacin 500 mg twice daily or amoxicillin plus clavulanic acid 375 mg three times daily for 7 or at least 14 days, respectively. Approximately 50% reductions in median trough MPA concentrations (pre-dose) from baseline (mycophenolate mofetil alone) were observed in 3 days following commencement of oral ciprofloxacin or amoxicillin plus clavulanic acid. These reductions in trough MPA concentrations tended to diminish within 14 days of antimicrobial therapy and ceased within 3 days of discontinuation of antibiotics.
•
Rifampin: In a single heart-lung transplant patient, after correction for dose, a 67% decrease in MPA exposure (AUC(0-12h)) has been observed with concomitant administration of MMF and rifampin.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Kidney Transplantation
Adults
The three de novo kidney transplantation studies compared two dose levels of oral mycophenolate mofetil (1 g twice daily and 1.5 g twice daily) with azathioprine (2 studies) or placebo (1 study) to prevent acute rejection episodes. One of the two studies with azathioprine (AZA) control arm also included anti-thymocyte globulin (ATGAM®) induction therapy. The geographic location of the investigational sites of these studies are included inTable 13.
In all three de novo kidney transplantation studies, the primary efficacy endpoint was the proportion of patients in each treatment group who experienced treatment failure within the first 6 months after transplantation. Treatment failure was defined as biopsy-proven acute rejection on treatment or the occurrence of death, graft loss or early termination from the study for any reason without prior biopsy-proven rejection.
Mycophenolate mofetil, in combination with corticosteroids and cyclosporine, reduced (statistically significant at 0.05 level) the incidence of treatment failure within the first 6 months following transplantation (Table 13). Patients who prematurely discontinued treatment were followed for the occurrence of death or graft loss, and the cumulative incidence of graft loss and patient death combined are summarized inTable 14. Patients who prematurely discontinued treatment were not followed for the occurrence of acute rejection after termination.
Table 13. Treatment Failure in De Novo Kidney Transplantation Studies
|
USA Study (N=499 patients) |
Mycophenolate mofetil (n=167 patients) |
Mycophenolate mofetil (n=166 patients) |
AZA (n=166 patients) |
|
All 3 groups received anti-thymocyte globulin induction, cyclosporine and corticosteroids | |||
|
All treatment failures |
31.1% |
31.3% |
47.6% |
|
Early termination without prior acute rejection |
9.6% |
12.7% |
6% |
|
Biopsy-proven rejection episode on treatment |
19.8% |
17.5% |
38% |
|
Europe/Canada/ Australia Study |
Mycophenolate mofetil (n=173 patients) |
Mycophenolate mofetil (n=164 patients) |
AZA |
|
No induction treatment administered; all 3 groups received cyclosporine and corticosteroids. | |||
|
All treatment failures |
38.2% |
34.8% |
50% |
|
Early termination without prior acute rejection |
13.9% |
15.2% |
10.2% |
|
Biopsy-proven rejection episode on treatment |
19.7% |
15.9% |
35.5% |
|
Europe Study (N=491 patients) |
Mycophenolate mofetil (n=165 patients) |
Mycophenolate mofetil (n=160 patients) |
Placebo (n=166 patients) |
|
No induction treatment administered; all 3 groups received cyclosporine and corticosteroids. | |||
|
All treatment failures |
30.3% |
38.8% |
56% |
|
Early termination without prior acute rejection |
11.5% |
22.5% |
7.2% |
|
Biopsy-proven rejection episode on treatment |
17% |
13.8% |
46.4% |
*Does not include death and graft loss as reason for early termination.
No advantage of mycophenolate mofetil at 12 months with respect to graft loss or patient death (combined) was established (Table 14). Numerically, patients receiving mycophenolate mofetil 2 g/day and 3 g/day experienced a better outcome than controls in all three studies; patients receiving mycophenolate mofetil 2 g/day experienced a better outcome than mycophenolate mofetil 3 g/day in two of the three studies. Patients in all treatment groups who terminated treatment early were found to have a poor outcome with respect to graft loss or patient death at 1 year.
**Table 14. De Novo Kidney Transplantation Studies Cumulative Incidence of Combined Graft Loss or Patient Death at 12 Months**
|
Study |
Mycophenolate mofetil |
Mycophenolate mofetil |
Control |
|
USA |
8.5% |
11.5% |
12.2% |
|
Europe/Canada/Australia |
11.7% |
11% |
13.6% |
|
Europe |
8.5% |
10% |
11.5% |
Pediatrics-De Novo Kidney transplantation PK Study with Long Term Follow- Up
One open-label, safety and pharmacokinetic study of mycophenolate mofetil oral suspension 600 mg/m2 twice daily (up to 1 g twice daily) in combination with cyclosporine and corticosteroids was performed at centers in the United States (9), Europe (5) and Australia (1) in 100 pediatric patients (3 months to 18 years of age) for the prevention of renal allograft rejection. Mycophenolate mofetil was well tolerated in pediatric patients [see Adverse Reactions (6.1)], and the pharmacokinetics profile was similar to that seen in adult patients dosed with 1 g twice daily mycophenolate mofetil capsules [see Clinical Pharmacology (12.3)]. The rate of biopsy-proven rejection was similar across the age groups (3 months to <6 years, 6 years to <12 years, 12 years to 18 years). The overall biopsy-proven rejection rate at 6 months was comparable to adults. The combined incidence of graft loss (5%) and patient death (2%) at 12 months post-transplant was similar to that observed in adult kidney transplant patients.
14.2 Heart Transplantation
A double-blind, randomized, comparative, parallel-group, multicenter study in primary de novo heart transplant recipients was performed at centers in the United States (20), in Canada (1), in Europe (5) and in Australia (2). The total number of patients enrolled (ITT population) was 650; 72 never received study drug and 578 received study drug (Safety Population). Patients received mycophenolate mofetil 1.5 g twice daily (n=289) or AZA 1.5 to 3 mg/kg/day (n=289), in combination with cyclosporine (Sandimmune® or Neoral®) and corticosteroids as maintenance immunosuppressive therapy. The two primary efficacy endpoints were: (1) the proportion of patients who, after transplantation, had at least one endomyocardial biopsy-proven rejection with hemodynamic compromise, or were re-transplanted or died, within the first 6 months, and (2) the proportion of patients who died or were re-transplanted during the first 12 months following transplantation. Patients who prematurely discontinued treatment were followed for the occurrence of allograft rejection for up to 6 months and for the occurrence of death for 1 year.
The analyses of the endpoints showed:
•
Rejection: No difference was established between mycophenolate mofetil and AZA with respect to biopsy-proven rejection with hemodynamic compromise.
•
Survival: Mycophenolate mofetil was shown to be at least as effective as AZA in preventing death or re-transplantation at 1 year (see**Table 15**).
Table 15. De Novo Heart Transplantation Study Rejection at 6 Months/Death or Re-transplantation at 1 Year
|
All Patients(ITT) |
Treated Patients | |||
|
AZA N=323 |
Mycophenolate mofetil N = 327 |
AZA N = 289 |
Mycophenolate mofetil N = 289 | |
|
Biopsy-proven rejection with hemodynamic compromise at 6 months* |
121 (38%) |
120 (37%) |
100 (35%) |
92 (32%) |
|
Death or re-transplantation at 1 year |
49 (15.2%) |
42 (12.8%) |
33 (11.4%) |
18 (6.2%) |
*Hemodynamic compromise occurred if any of the following criteria were met: pulmonary capillary wedge pressure ≥20 mm or a 25% increase; cardiac index <2 L/min/m2 or a 25% decrease; ejection fraction ≤30%; pulmonary artery oxygen saturation ≤60% or a 25% decrease; presence of new S3 gallop; fractional shortening was ≤20% or a 25% decrease; inotropic support required to manage the clinical condition.
14.3 Liver Transplantation
A double-blind, randomized, comparative, parallel-group, multicenter study in primary hepatic transplant recipients was performed at centers in the United States (16), in Canada (2), in Europe (4) and in Australia (1). The total number of patients enrolled was 565. Per protocol, patients received mycophenolate mofetil 1 g twice daily intravenously for up to 14 days followed by mycophenolate mofetil 1.5 g twice daily orally or AZA 1 to 2 mg/kg/day intravenously followed by AZA 1 to 2 mg/kg/day orally, in combination with cyclosporine (Neoral®) and corticosteroids as maintenance immunosuppressive therapy. The actual median oral dose of AZA on study was 1.5 mg/kg/day (range of 0.3 to 3.8 mg/kg/day) initially and 1.26 mg/kg/day (range of 0.3 to 3.8 mg/kg/day) at 12 months. The two primary endpoints were: (1) the proportion of patients who experienced, in the first 6 months post-transplantation, one or more episodes of biopsy-proven and treated rejection or death or re- transplantation, and (2) the proportion of patients who experienced graft loss (death or re-transplantation) during the first 12 months post-transplantation. Patients who prematurely discontinued treatment were followed for the occurrence of allograft rejection and for the occurrence of graft loss (death or re-transplantation) for 1 year.
In combination with corticosteroids and cyclosporine, mycophenolate mofetil demonstrated a lower rate of acute rejection at 6 months and a similar rate of death or re-transplantation at 1 year compared to AZA (Table 16).
Table 16. De Novo Liver Transplantation Study Rejection at 6 Months/Death or Retransplantation at 1 Year
|
AZA |
Mycophenolate mofetil | |
|
Biopsy-proven, treated rejection at 6 months (includes death or re- transplantation) |
137 (47.7%) |
107 (38.5%) |
|
Death or re-transplantation at 1 year |
42 (14.6%) |
41 (14.7%) |
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warnings and Precautions (5.8)……………………………………….05/2025
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Mycophenolate mofetil should not be used without the supervision of a physician with experience in immunosuppressive therapy.
Mycophenolate Mofetil Capsules and Tablets
Mycophenolate mofetil oral dosage forms (capsules or tablets)**should not be used interchangeably with mycophenolic acid delayed-release tablets without supervision of a physician with experience in immunosuppressive therapy **because the rates of absorption following the administration of mycophenolate mofetil oral dosage forms and mycophenolic acid delayed-release tablets are not equivalent.
Mycophenolate mofetil tablets should not be crushed and mycophenolate mofetil capsules should not be opened or crushed. Patients should avoid inhalation or contact of the skin or mucous membranes with the powder contained in mycophenolate mofetil capsules. If such contact occurs, they must wash the area of contact thoroughly with soap and water. In case of ocular contact, rinse eyes with plain water.
The initial oral dose of mycophenolate mofetil should be given as soon as possible following kidney, heart or liver transplant. It is recommended that mycophenolate mofetil be administered on an empty stomach. In stable transplant patients, however, mycophenolate mofetil may be administered with food if necessary [see Clinical Pharmacology (12.3)].
Patients should be instructed to take a missed dose as soon as they remember, except if it is closer than 2 hours to the next scheduled dose; in this case, they should continue to take mycophenolate mofetil at the usual times.
2.2 Dosage Recommendations for Kidney Transplant Patients
Adults
The recommended dosage for adult kidney transplant patients is 1 g orally or intravenously infused over no less than 2 hours, twice daily (total daily dose of 2 g).
Pediatrics (3 months and older)
Pediatric dosing is based on body surface area (BSA). The recommended dosage of mycophenolate mofetil oral suspension for pediatric kidney transplant patients 3 months and older is 600 mg/m2, administered twice daily (maximum total daily dose of 2 g or 10 mL of the oral suspension). Pediatric patients with BSA ≥1.25 m2 may be dosed with capsules or tablets as follows:
Table 1. Pediatric Kidney Transplant: Dosage Using Capsules or Tablets
|
Body Surface Area |
Dosage |
|
1.25 m2 to <1.5 m2 |
Mycophenolate mofetil capsule 750 mg twice daily (1.5 g total daily dose) |
|
≥1.5 m2 |
Mycophenolate mofetil capsules or tablets 1 g twice daily (2 g total daily dose) |
2.3 Dosage Recommendations for Heart Transplant Patients
Adults
The recommended dosage of mycophenolate mofetil for adult heart transplant patients is 1.5 g orally or intravenously infused over no less than 2 hours administered twice daily (total daily dose of 3 g).
Pediatrics (3 months and older)
The recommended starting dosage of mycophenolate mofetil oral suspension for pediatric heart transplant patients 3 months and older is 600 mg/m2, administered twice daily. If well tolerated, the dose can be increased to a maintenance dosage of 900 mg/m2 twice daily (maximum total daily dose of 3 g or 15 mL of the oral suspension). The dose may be individualized based on clinical assessment.
Pediatric patients with BSA ≥1.25 m2 may be started on therapy with capsules or tablets as follows:
Table 2. Pediatric Heart Transplant: Pediatric Starting Dosage Using Capsules or Tablets
|
Body Surface Area |
Starting Dosage* |
|
1.25 m2 to <1.5 m2 |
Mycophenolate mofetil capsule 750 mg twice daily (1.5 g total daily dose) |
|
≥1.5 m2 |
Mycophenolate mofetil capsules or tablets 1 g twice daily (2 g total daily dose) |
*Maximum maintenance dose: 3 g total daily.
2.4 Dosage Recommendations for Liver Transplant Patients
Adults
The recommended dosage of mycophenolate mofetil for adult liver transplant patients is 1.5 g administered orally twice daily (total daily dose of 3 g) or 1 g infused intravenously over no less than 2 hours, twice daily (total daily dose of 2 g).
Pediatrics (3 months and older)
The recommended starting dosage of mycophenolate mofetil oral suspension for pediatric liver transplant patients 3 months of age and older is 600 mg/m2, administered twice daily. If well tolerated, the dose can be increased to a maintenance dosage of 900 mg/m2 twice daily (maximum total daily dose of 3 g or 15 mL of the oral suspension). The dose may be individualized based on clinical assessment.
Pediatric patients with BSA ≥1.25 m2 may be started on therapy with capsules or tablets as follows:
Table 3. Pediatric Liver Transplant: Pediatric Starting Dosage Using Capsules or Tablets
|
Body Surface Area |
Starting Dosage* |
|
1.25 m2 to <1.5 m2 |
Mycophenolate mofetil capsule 750 mg twice daily (1.5 g total daily dose) |
|
≥1.5 m2 |
Mycophenolate mofetil capsules or tablets 1 g twice daily (2 g total daily dose) |
*Maximum maintenance dose: 3 g total daily.
2.5 Dosage Modifications: Patients with Renal Impairment, Neutropenia
Renal Impairment
No dosage modifications are needed in kidney transplant patients with delayed graft function postoperatively [see Clinical Pharmacology (12.3)]. In kidney transplant patients with severe chronic impairment of the graft (GFR <25 mL/min/1.73 m2), do not administer doses of mycophenolate mofetil greater than 1 g twice a day. These patients should be carefully monitored [see Clinical Pharmacology (12.3)].
Neutropenia
If neutropenia develops (ANC <1.3 x 103/µL), dosing with mycophenolate mofetil should be interrupted or reduced, appropriate diagnostic tests performed, and the patient managed appropriately [see Warnings and Precautions (5.4) and Adverse Reactions (6.1)].
|
ADULTS |
DOSAGE |
|
Kidney Transplant |
1 g twice daily, orally or intravenously (IV) over no less than 2 h (2.2) |
|
Heart Transplant |
1.5 g twice daily orally or IV, over no less than 2 h (2.3) |
|
Liver Transplant |
1.5 g twice daily orally or 1 g twice daily IV over no less than 2 h (2.4) |
|
PEDIATRICS | |
|
Kidney Transplant |
600 mg/m2 orally twice daily, up to maximum of 2 g daily (2.2) |
|
Heart Transplant |
600 mg/m2 orally twice daily (starting dose) up to a maximum of 900 mg/m2 twice daily (3 g or 15 mL of oral suspension) (2.3) |
|
Liver Transplant |
600 mg/m2 orally twice daily (starting dose) up to a maximum of 900 mg/m2 twice daily (3 g or 15 mL of oral suspension) (2.4) |
•
Mycophenolate mofetil Intravenous is an alternative when patients cannot tolerate oral medication. Administer within 24 hours following transplantation, until patients can tolerate oral medication, up to 14 days. (2.1)
•
Reduce or interrupt dosing in the event of neutropenia. (2.5)
•
See full prescribing information (FPI) for: adjustments for renal impairment and neutropenia (2.5).
DESCRIPTION SECTION
11 DESCRIPTION
Mycophenolate mofetil is an antimetabolite immunosuppressant. It is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor.
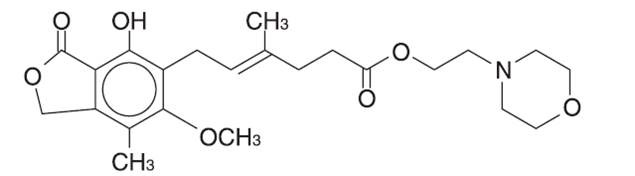
The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate. It has a molecular formula of C23H31NO7, a molecular weight of 433.50, and the following structural formula:
Mycophenolate mofetil is a white to off-white crystalline powder. It is slightly soluble in water (43 mcg/mL at pH 7.4); the solubility increases in acidic medium (4.27 mg/mL at pH 3.6). It is freely soluble in acetone, soluble in methanol, and sparingly soluble in ethanol. The apparent partition coefficient in 1-octanol/water (pH 7.4) buffer solution is 238. The pKa values for mycophenolate mofetil are 5.6 for the morpholino group and 8.5 for the phenolic group.
Inactive Ingredients:
Tablets - Mycophenolate mofetil tablets are available for oral administration as tablets containing 500 mg of mycophenolate mofetil. In addition, each tablet contains the following inactive ingredients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, povidone, talc, hypromellose, hydroxypropyl cellulose, polyethylene glycol, titanium dioxide, iron oxide black and iron oxide red.
**Capsules -**Mycophenolate mofetil capsules, USP are available for oral administration as capsules containing 250 mg of mycophenolate mofetil. In addition, each capsule contains the following inactive ingredients: croscarmellose sodium, FD&C blue #2, gelatin, magnesium stearate, povidone (K-90), pregelatinized starch and titanium dioxide. The capsule is printed with edible black ink. The black ink is comprised of butyl alcohol, black iron oxide, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, shellac and strong ammonia solution.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Handling and Disposal
Mycophenolate mofetil (MMF) has demonstrated teratogenic effects in humans [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)]. Mycophenolate mofetil tablets should not be crushed and mycophenolate mofetil capsules should not be opened or crushed. Avoid inhalation or direct contact with skin or mucous membranes of the powder contained in mycophenolate mofetil capsules. Follow applicable special handling and disposal procedures1.
16.2 Mycophenolate Mofetil Capsules, 250 mg
Mycophenolate mofetil capsules, USP are available as follows:
Capsules
Hard gelatin capsules with opaque blue cap and opaque white body. The cap and body are imprinted with "655" with black ink
NDC 0781-2067-01, bottle of 100 capsules
NDC 0781-2067-72, bottle of 120 capsules
NDC 0781-2067-05, bottle of 500 capsules
NDC 0781-2067-89, package containing 12 bottles of 120
16.3 Mycophenolate Mofetil Tablets, 500 mg
Mycophenolate mofetil tablets are available as follows:
Tablets
Lavender colored, film-coated biconvex tablets with ‘SZ’ on one side and ‘327’ on the other side.
NDC 0781-5175-01, bottle of 100 tablets
NDC 0781-5175-05, bottle of 500 tablets
NDC 0781-5175-10, bottle of 1000 tablets
Storage:
Store at 20ºC to 25°C (68ºF to 77°F) [see USP Controlled Room Temperature]. Protect from moisture. Dispense in a tight, light-resistant container as defined in the USP with a child-resistant cap.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
17.1 Embryofetal Toxicity
Pregnancy loss and malformations
•
Inform females of reproductive potential and pregnant women that use of mycophenolate mofetil during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations. Advise that they must use an acceptable form of contraception [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
•
Encourage pregnant women to enroll in the Pregnancy Exposure Registry. This registry monitors pregnancy outcomes in women exposed to mycophenolate [see Use in Specific Populations (8.1)].
Contraception
•
Discuss pregnancy testing, pregnancy prevention and planning with females of reproductive potential [see Use in Specific Populations (8.3)].
•
Females of reproductive potential must use an acceptable form of birth control during the entire mycophenolate mofetil therapy and for 6 weeks after stopping mycophenolate mofetil, unless the patient chooses abstinence. Mycophenolate mofetil may reduce effectiveness of oral contraceptives. Use of additional barrier contraceptive methods is recommended [see Use in Specific Populations (8.3)].
•
For patients who are considering pregnancy, discuss appropriate alternative immunosuppressants with less potential for embryofetal toxicity. Risks and benefits of mycophenolate mofetil should be discussed with the patient.
•
Advise sexually active male patients and/or their partners to use effective contraception during the treatment of the male patient and for at least 90 days after cessation of treatment. This recommendation is based on findings of animal studies [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
17.2 Development of Lymphoma and Other Malignancies
•
Inform patients that they are at increased risk of developing lymphomas and other malignancies, particularly of the skin, due to immunosuppression [see Warnings and Precautions (5.2)].
•
Advise patients to limit exposure to sunlight and ultraviolet (UV) light by wearing protective clothing and use of broad-spectrum sunscreen with high protection factor.
17.3 Increased Risk of Serious Infections
Inform patients that they are at increased risk of developing a variety of infections due to immunosuppression. Instruct them to contact their physician if they develop any of the signs and symptoms of infection explained in the Medication Guide [see Warnings and Precautions (5.3)].
17.4 Blood Dyscrasias
Inform patients that they are at increased risk for developing blood adverse effects such as anemia or low white blood cells. Advise patients to immediately contact their healthcare provider if they experience any evidence of infection, unexpected bruising, or bleeding, or any other manifestation of bone marrow suppression [see Warnings and Precautions (5.4)].
17.5 Gastrointestinal Tract Complications
Inform patients that mycophenolate mofetil can cause gastrointestinal tract complications including bleeding, intestinal perforations, and gastric or duodenal ulcers. Advise the patient to contact their healthcare provider if they have symptoms of gastrointestinal bleeding, or sudden onset or persistent abdominal pain [see Warnings and Precautions (5.5)].
17.6 Acute Inflammatory Syndrome
Inform patients that acute inflammatory reactions have been reported in some patients who received mycophenolate mofetil. Some reactions were severe, requiring hospitalization. Advise patients to contact their physician if they develop fever, joint stiffness, joint pain or muscle pains [see Warnings and Precautions (5.7)].
17.7 Hypersensitivity Reactions
Inform patients of the potential risk of hypersensitivity reactions. Advise patients to stop taking mycophenolate mofetil and seek immediate medical attention if signs or symptoms of hypersensitivity reaction occur (such as swelling of face, lips, tongue, or throat; difficulty breathing or swallowing) [see Warnings and Precautions (5.8)].
17.8 Immunizations
Inform patients that mycophenolate mofetil can interfere with the usual response to immunizations. Before seeking vaccines on their own, advise patients to discuss first with their physician [see Warnings and Precautions (5.9)].
17.9 Administration Instructions
•
Advise patients not to crush mycophenolate mofetil tablets and not to open mycophenolate mofetil capsules.
•
Advise patients to avoid inhalation or contact of the skin or mucous membranes with the powder contained in mycophenolate mofetil capsules. If such contact occurs, they must wash the area of contact thoroughly with soap and water. In case of ocular contact, rinse eyes with plain water.
•
Advise patients to take a missed dose as soon as they remember, except if it is closer than 2 hours to the next scheduled dose; in this case they should continue to take mycophenolate mofetil at the usual times.
17.10 Blood Donation
Advise patients not to donate blood during therapy and for at least 6 weeks following discontinuation of mycophenolate mofetil [see Warnings and Precautions (5.12)].
17.11 Semen Donation
Advise males of childbearing potential not to donate semen during therapy and for 90 days following discontinuation of mycophenolate mofetil [see Warnings and Precautions (5.13)].
17.12 Potential to Impair Driving and Use of Machinery
Advise patients that mycophenolate mofetil can affect the ability to drive or operate machines. Patients should avoid driving or operating machines if they experience somnolence, confusion, dizziness, tremor or hypotension during treatment with mycophenolate mofetil [see Warnings and Precautions (5.15)].
Manufactured by Sandoz Private Limited, India for
Sandoz Inc., Princeton, NJ 08540
SPL MEDGUIDE SECTION
|
MEDICATION GUIDE Mycophenolate Mofetil Capsules and Tablets (mye-koe-FIN-oh-late) |
|
Read the Medication Guide that comes with mycophenolate mofetil before you start taking it and each time you refill your prescription. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or treatment. |
|
What is the most important information I should know about mycophenolate mofetil? Mycophenolate mofetil can cause serious side effects, including: Increased risk of loss of a pregnancy (miscarriage) and higher risk of birth defects. Females who take mycophenolate mofetil during pregnancy have a higher risk of miscarriage during the first 3 months (first trimester), and a higher risk that their baby will be born with birth defects. • • • • • o o Increased risk of getting certain cancers. People who take mycophenolate mofetil have a higher risk of getting lymphoma, and other cancers, especially skin cancer. Tell your doctor if you have: • • • • • Increased risk of getting serious infections. Mycophenolate mofetil weakens the body’s immune system and affects your ability to fight infections. Serious infections can happen with mycophenolate mofetil and can lead to hospitalizations and death. These serious infections can include: • o o o o • o o o o • Call your doctor right away if you have any of the following signs and symptoms of infection: • • • • • • • • See**“What are the possible side effects of mycophenolate mofetil?”** for information about other serious side effects. |
|
What is mycophenolate mofetil? • • |
|
Who should not take mycophenolate mofetil? Do not take mycophenolate mofetil if you have a history of allergic reactions to mycophenolate mofetil or any of the ingredients in mycophenolate mofetil. See the end of this Medication Guide for a complete list of ingredients in mycophenolate mofetil. |
|
What should I tell my doctor before taking mycophenolate mofetil? Tell your doctor about all of your medical conditions, including if you: • • • • • Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Some medicines may affect the way mycophenolate mofetil works, and mycophenolate mofetil may affect how some medicines work. Especially tell your doctor if you take: • • • • • • • • • • • Know the medicines you take. Keep a list of them to show to your doctor or nurse and pharmacist when you get a new medicine. Do not take any new medicine without talking with your doctor. |
|
How should I take mycophenolate mofetil? • • • • • • o o • |
|
What should I avoid while taking mycophenolate mofetil? • • • • • |
|
What are the possible side effects of mycophenolate mofetil? Mycophenolate mofetil may cause serious side effects, including: • • o o o • • • • • • • • • The most common side effects of mycophenolate mofetil include: • • • • • • • • • • Side effects that can happen more often in children than in adults taking mycophenolate mofetil include: • • • • • • • • • • • • These are not all of the possible side effects of mycophenolate mofetil. Tell your doctor about any side effect that bothers you or that does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Sandoz Inc. at 1-800-525-8747. |
|
How should I store mycophenolate mofetil? • • Keep mycophenolate mofetil and all medicines out of the reach of children. |
|
General information about the safe and effective use of mycophenolate mofetil. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use mycophenolate mofetil for a condition for which it was not prescribed. Do not give mycophenolate mofetil to other people, even if they have the same symptoms that you have. It may harm them. This Medication Guide summarizes the most important information about mycophenolate mofetil. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist about mycophenolate mofetil that is written for health professionals. |
|
What are the ingredients in mycophenolate mofetil? Active ingredient: mycophenolate mofetil Inactive ingredients: Mycophenolate mofetil 250 mg capsules: croscarmellose sodium, FD&C blue #2, gelatin, magnesium stearate, povidone (K-90), pregelatinized starch and titanium dioxide. The capsule is printed with edible black ink. The black ink is comprised of butyl alcohol, black iron oxide, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, shellac and strong ammonia solution. Mycophenolate mofetil 500 mg tablets: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, povidone, talc, hypromellose, hydroxypropyl cellulose, polyethylene glycol, titanium dioxide, iron oxide black and iron oxide red. The brands listed are trademark of their respective owners and are not trademark of Sandoz Inc. For additional medication guide please go to www.us.sandoz.com or call 1-800-525-8747. Manufactured by Sandoz Private Limited, India for Sandoz Inc., Princeton, NJ 08540 |
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 07/2025
