New Terocin
New Terocin Topical Pain Relief Lotion
186445d4-ef73-4d34-849d-4661a3eaa43a
HUMAN OTC DRUG LABEL
Sep 30, 2025
Preferred Pharmaceuticals Inc.
DUNS: 791119022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Methyl Salicylate, Capsaicin, and Menthol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (21)
Drug Labeling Information
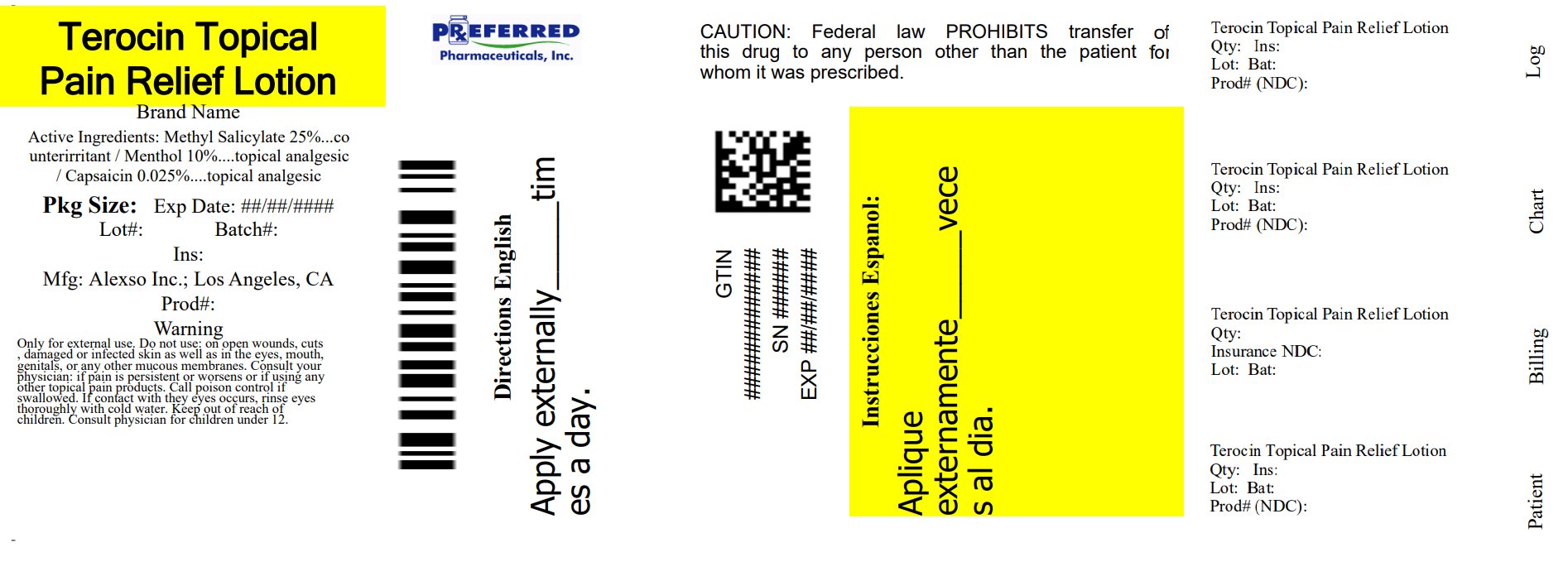
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Principal Display Panel
NDC 68788-7821-1
New Terocin
Topical Pain
Relief Lotion
Long Lasting
Soothing Effect
120 ml (4 fl oz.)
Manufactured for: Alexso Inc.
Los Angeles, CA 90064
Store in a dry, cool place
Made in U.S.A.
Patent Pending
For Comments or Questions,
call 888-495-6075
Relabeled By: Preferred Pharmaceuticals Inc.
Label
INDICATIONS & USAGE SECTION
Uses:
Temporarily relieves mild aches and pains of muscles or joints.
INACTIVE INGREDIENT SECTION
Inactive Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Borago Officinalis (Borage) Seed Oil, Boswellia Serrata Extract, C12-15 Alkyl Benzoate, Cetearyl Alcohol, Cetyl Alcohol, Diazolidinyl Urea, Dimethyl Sulfone (DMSO), DMDM Hydantoin, Glyceryl Stearate, Lavandula Angustifolia (Lavender) Oil, Methyl Paraben, PEG-100 Stearate, Polysorbate-20, Polysorbate-60, Propyl Paraben, Propylene Glycol, Stearic Acid, Stearyl Alcohol, Triethanolamine, Xanthan Gum.
DOSAGE & ADMINISTRATION SECTION
Directions:
Wash and dry affected area. Shake bottle well before each use and gently rub over area of pain. Use is not recommended more than four times a day. Wash hands immediately afterwards to avoid contact with eyes.
WARNINGS SECTION
Warnings
Only for external use.
Do Not Use:
on open wounds, cuts, damaged or infected skin as well as in the eyes, mouth, genitals, or any other mucous membranes.
Consult your physician:
if pain is persistent or worsens or if using any other topical pain products.
Keep out of reach of children.
Consult physician for children under 12.
OTC - ACTIVE INGREDIENT SECTION
Active ingredients:
Methyl Salicylate 25%
Capsaicin 0.025%
Menthol 10%
OTC - PURPOSE SECTION
Purpose
Topical Analgesic
