Methylphenidate
These highlights do not include all the information needed to use METHYLPHENIDATE HYDROCHLORIDE EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for METHYLPHENIDATE HYDROCHLORIDE EXTENDED-RELEASE CAPSULES. METHYLPHENIDATE HYDROCHLORIDE Extended-Release Capsules for oral use, CIIInitial U.S. Approval: 1955
1f8983ce-71b8-4c62-830d-e4692ddededa
HUMAN PRESCRIPTION DRUG LABEL
Feb 28, 2024
Sandoz Inc
DUNS: 005387188
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
methylphenidate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
methylphenidate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
methylphenidate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
methylphenidate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0781-2364-01 Rx only
Methylphenidate HCl Extended-Release Capsules
40 mg
100 capsules
Dispense with Medication
Guide attached or provided
separately.
SANDOZ

Boxed Warning section
WARNING: ABUSE, MISUSE, AND ADDICTION
See full prescribing information for complete boxed warning.
Methylphenidate hydrochloride extended-release capsules****have a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including methylphenidate hydrochloride extended-release capsules, can result in overdose and death (5.1,9.2,10).
•
**Before prescribing methylphenidate hydrochloride extended-release capsules, assess each patient’s risk for abuse, misuse, and addiction.**
•
**Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug.**
•
**Throughout treatment, reassess each patient’s risk and frequently monitor for signs and symptoms of abuse, misuse, and addiction.**
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Methylphenidate hydrochloride extended-release capsules are indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD), in pediatric patients 6 to 12 years of age [see Clinical Studies (14)].
Methylphenidate hydrochloride extended-release capsules are a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in pediatric patients 6 to 12 years of age (1).
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
•
Hypersensitivity to methylphenidate or other components of methylphenidate hydrochloride extended-release capsules. Hypersensitivity reactions, such as angioedema and anaphylactic reactions, have been reported in patients treated with methylphenidate [see Adverse Reactions (6.1)].
•
Concomitant treatment with monoamine oxidase inhibitors (MAOIs), or within 14 days following discontinuation of treatment with an MAOI, because of the risk of hypertensive crises [see Drug Interactions (7.1)].
•
Known hypersensitivity to methylphenidate or product components (4).
•
Concurrent treatment with a monoamine oxidase inhibitor (MAOI) or use of an MAOI within the preceding 14 days (4).
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
•
Abuse, Misuse, and Addiction [see Boxed Warning, Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2, 9.3)]
•
Known hypersensitivity to methylphenidate or other ingredients of methylphenidate hydrochloride extended-release capsules [see Contraindications (4)]
•
Hypertensive crisis when used concomitantly with Monoamine Oxidase Inhibitors [see Contraindications (4), Drug Interactions (7.1)]
•
Risks to Patients with Serious Cardiac Disease [see Warnings and Precautions (5.2)]
•
Increased Blood Pressure and Heart Rate [see Warnings and Precautions (5.3)]
•
Psychiatric Adverse Reactions [see Warnings and Precautions (5.4)]
•
Priapism [see Warnings and Precautions (5.5)]
•
Peripheral Vasculopathy, Including Raynaud’s Phenomenon [see Warnings and Precautions (5.6)]
•
Long-Term Suppression of Growth in Pediatric Patients [see Warnings and Precautions (5.7)]
•
Acute Angle Closure Glaucoma [see Warnings and Precautions (5.8)]
•
Increased Intraocular Pressure and Glaucoma [see Warnings and Precautions (5.9)]
•
Motor and Verbal Tics, and Worsening of Tourette’s Syndrome [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The clinical program for methylphenidate hydrochloride extended-release capsules consisted of 6 studies: 2 controlled clinical studies conducted in children with ADHD aged 6 to 12 years and 4 clinical pharmacology studies conducted in healthy adult volunteers. These studies included a total of 256 subjects; 195 children with ADHD and 61 healthy adult volunteers. The subjects received methylphenidate hydrochloride extended-release capsules in doses of 10 to 40 mg per day. Safety of methylphenidate hydrochloride extended-release capsules was assessed by evaluating frequency and nature of adverse events, routine laboratory tests, vital signs, and body weight. A placebo-controlled, double-blind, parallel-group study was conducted to evaluate the efficacy and safety of methylphenidate hydrochloride extended-release capsules in children with ADHD aged 6 to 12 years. All subjects received methylphenidate hydrochloride extended-release capsules for up to 4 weeks, and had their dose optimally adjusted, prior to entering the double-blind phase of the trial. In the 2-week double-blind treatment phase of this study, patients received either placebo or methylphenidate hydrochloride extended-release capsules at their individually-titrated dose (range, 10 mg to 40 mg).
Adverse reactions with an incidence greater than 5% during the initial 4-week single-blind methylphenidate hydrochloride extended-release capsules titration period of this study were headache, insomnia, upper abdominal pain, appetite decreased, and anorexia.
Adverse reactions with an incidence greater than 2% among methylphenidate hydrochloride extended-release capsules-treated subjects, during the 2-week double-blind phase of the clinical study, are shown in Table 2.
Table 2: Adverse Reactions in Greater Than 2% Methylphenidate Hydrochloride Extended-Release Capsules-Treated Subjects in the 2-Week Double- Blind Phase|
Preferred Term |
Methylphenidate Hydrochloride Extended-Release Capsules N = 65 |
Placebo |
|
Anorexia |
2 (3.1) |
0 (0.0) |
|
Insomnia |
2 (3.1) |
0 (0.0) |
Adverse Reactions Associated with Discontinuation of Treatment
In the 2-week double-blind treatment phase of a placebo-controlled parallel- group study in children with ADHD, one methylphenidate hydrochloride extended- release capsules-treated subject (1/65, 1.5%) discontinued due to an adverse event (depressed mood).
In the single-blind titration period of this study, subjects received methylphenidate hydrochloride extended-release capsules for up to 4 weeks. During this period a total of 6 subjects (6/161, 3.7%) discontinued due to adverse events. The adverse events leading to discontinuation were anger (2 patients), hypomania, anxiety, depressed mood, fatigue, migraine, and lethargy.
6.2 Postmarketing Experience
The following adverse reactions have been identified during the post approval use of methylphenidate products. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Adverse Reactions Reported with Methylphenidate Hydrochloride Tablets and Methylphenidate Hydrochloride Extended-Release Capsules
Infections and Infestations: nasopharyngitis
Blood and the Lymphatic System Disorders: leukopenia, thrombocytopenia, anemia
Immune System Disorders: hypersensitivity reactions, including angioedema and anaphylaxis
Metabolism and Nutrition Disorders: decreased appetite, reduced weight gain, and suppression of growth during prolonged use in children
Psychiatric Disorders: insomnia, anxiety, restlessness, agitation, psychosis (sometimes with visual and tactile hallucinations), depressed mood, depression
Nervous System Disorders: headache, dizziness, tremor, dyskinesia, including choreoathetoid movements, drowsiness, convulsions, cerebrovascular disorders (including vasculitis, cerebral hemorrhages and cerebrovascular accidents), serotonin syndrome in combination with serotonergic drugs
Eye Disorders: blurred vision, difficulties in visual accommodation
Cardiac Disorders: tachycardia, palpitations, increased blood pressure, arrhythmias, angina pectoris
Respiratory, Thoracic, and Mediastinal Disorders: cough
Gastrointestinal Disorders: dry mouth, nausea, vomiting, abdominal pain, dyspepsia
Hepatobiliary Disorders: abnormal liver function, ranging from transaminase elevation to severe hepatic injury
Skin and Subcutaneous Tissue Disorders: hyperhidrosis, pruritus, urticaria, exfoliative dermatitis, scalp hair loss, erythema multiforme rash, thrombocytopenic purpura
Musculoskeletal and Connective Tissue Disorders: arthralgia, muscle cramps, rhabdomyolysis, trismus
Investigations: weight loss (adult ADHD patients)
Vascular Disorders: peripheral coldness, Raynaud's phenomenon
Adverse Reactions Reported with Other Methylphenidate-Containing Products
The list below shows adverse reactions not listed with methylphenidate hydrochloride tablets or methylphenidate hydrochloride extended-release capsules formulations that have been reported with other methylphenidate- containing products.
Blood and Lymphatic Disorders: pancytopenia
Immune System Disorders: hypersensitivity reactions, such as auricular swelling, bullous conditions, eruptions, exanthemas
Psychiatric Disorders: affect lability, mania, disorientation, libido changes
Nervous System Disorders: migraine, motor and verbal tics
Eye Disorders: diplopia, increased intraocular pressure, mydriasis
Cardiac Disorders: sudden cardiac death, myocardial infarction, bradycardia, extrasystole
Respiratory, Thoracic, and Mediastinal Disorders: pharyngolaryngeal pain, dyspnea
Gastrointestinal Disorders: diarrhea, constipation
Skin and Subcutaneous Tissue Disorders: angioneurotic edema, erythema, fixed drug eruption
Musculoskeletal, Connective Tissue, and Bone Disorders: myalgia, muscle twitching
Renal and Urinary Disorders: hematuria
Reproductive System and Breast Disorders: gynecomastia
General Disorders: fatigue, hyperpyrexia
Urogenital Disorders: priapism
Most common adverse reactions (greater than 5% during incidence) were headache, insomnia, upper abdominal pain, decreased appetite, and anorexia (6).
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc., at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Clinically Important Drug Interactions with Methylphenidate
Hydrochloride Extended-Release Capsules
Table 3 presents clinically important drug interactions with methylphenidate hydrochloride extended-release capsules.
Table 3: Clinically Important Drug Interactions with Methylphenidate Hydrochloride Extended-Release Capsules|
Monoamine Oxidase Inhibitors (MAOI) | |
|
Clinical impact |
Concomitant use of MAOIs and CNS stimulants, including methylphenidate hydrochloride extended-release capsules, can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure [see Contraindications (4)]. |
|
Intervention |
Concomitant use of methylphenidate hydrochloride extended-release capsules with MAOIs or within 14 days after discontinuing MAOI treatment is contraindicated. |
|
Antihypertensive Drugs | |
|
Clinical impact |
Methylphenidate hydrochloride extended-release capsules may decrease the effectiveness of drugs used to treat hypertension [see Warnings and Precautions (5.3)]. |
|
Intervention |
Monitor blood pressure and adjust the dosage of the antihypertensive drug as needed. |
|
Halogenated Anesthetics | |
|
Clinical impact |
Concomitant use of halogenated anesthetics and methylphenidate hydrochloride extended-release capsules may increase the risk of sudden blood pressure and heart rate increase during surgery. |
|
Intervention |
Avoid use of methylphenidate hydrochloride extended-release capsules in patients being treated with anesthetics on the day of surgery. |
|
Risperidone | |
|
Clinical impact |
Combined use of methylphenidate with risperidone when there is a change, whether an increase or decrease, in dosage of either or both medications, may increase the risk of extrapyramidal symptoms (EPS) |
|
Intervention |
Monitor for signs of EPS |
•
Antihypertensive Drugs: Monitor blood pressure and heart. Adjust dosage of antihypertensive drug as needed (7.1).
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including methylphenidate hydrochloride extended-release capsules during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for ADHD medications at 1-866-961-2388 or visiting https://womensmentalhealth.org/adhd-medications/.
Risk Summary
Published studies and postmarketing reports on methylphenidate use during pregnancy have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There may be risks to the fetus associated with the use of CNS stimulants use during pregnancy (see Clinical Considerations).
No effects on morphological development were observed in embryo-fetal development studies with oral administration of methylphenidate to pregnant rats and rabbits during organogenesis at doses up to 10 and 15 times, respectively, the maximum recommended human dose (MRHD) of 60 mg/day given to adolescents on a mg/m2 basis. However, spina bifida was observed in rabbits at a dose 52 times the MRHD given to adolescents. A decrease in pup body weight was observed in a pre- and post-natal development study with oral administration of methylphenidate to rats throughout pregnancy and lactation at doses 6 times the MRHD given to adolescents (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
CNS stimulants, such as methylphenidate hydrochloride extended-release capsules, can cause vasoconstriction and thereby decrease placental perfusion. No fetal and/or neonatal adverse reactions have been reported with the use of therapeutic doses of methylphenidate during pregnancy; however, premature delivery and low birth-weight-infants have been reported in amphetamine- dependent mothers.
Data
Animal Data
In embryo-fetal development studies conducted in rats and rabbits, methylphenidate was administered orally at doses of up to 75 and 200 mg/kg/day, respectively, during the period of organogenesis. Malformations (increased incidence of fetal spina bifida) were observed in rabbits at the highest dose, which is approximately 52 times the MRHD of 60 mg/day given to adolescents on a mg/m2 basis. The no effect level for embryo-fetal development in rabbits was 60 mg/kg/day (15 times the MRHD given to adolescents on a mg/m2 basis). There was no evidence of morphological development effects in rats, although increased incidences of fetal skeletal variations were seen at the highest dose level (10 times the MRHD of 60 mg/day given to adolescents on a mg/m2 basis), which was also maternally toxic. The no effect level for embryo- fetal development in rats was 25 mg/kg/day (3 times the MRHD on a mg/m2 basis). When methylphenidate was administered to rats throughout pregnancy and lactation at doses of up to 45 mg/kg/day, offspring body weight gain was decreased at the highest dose (6 times the MRHD of 60 mg/day given to adolescents on a mg/m2 basis), but no other effects on postnatal development were observed. The no effect level for pre- and post-natal development in rats was 15 mg/kg/day (approximately 2 times the MRHD given to adolescents on a mg/m2 basis).
8.2 Lactation
Risk Summary
Limited published literature, based on milk sampling from seven mothers reports that methylphenidate is present in human milk, which resulted in infant doses of 0.16% to 0.7% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.1 and 2.7. There are no reports of adverse effects on the breastfed infant and no effects on milk production. Long-term neurodevelopmental effects on infants from stimulant exposure are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for methylphenidate hydrochloride extended- release capsules and any potential adverse effects on the breastfed infant from methylphenidate hydrochloride extended-release capsules or from the underlying maternal condition.
Clinical Considerations
Monitor breastfeeding infants for adverse reactions, such as agitation, insomnia, anorexia, and reduced weight gain.
8.4 Pediatric Use
The safety and effectiveness of methylphenidate hydrochloride extended-release capsules for the treatment of ADHD have been established in pediatric patients aged 6 to 12 years.
The safety and effectiveness of methylphenidate hydrochloride extended-release capsules in pediatric patients aged less than 6 years have not been established.
The long-term efficacy of methylphenidate hydrochloride extended-release capsules in pediatric patients has not been established.
Long-Term Suppression of Growth
Growth should be monitored during treatment with stimulants, including methylphenidate hydrochloride extended-release capsules. Pediatric patients who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.7)].
Juvenile Animal Toxicity Data
Rats treated with methylphenidate early in the postnatal period through sexual maturation demonstrated a decrease in spontaneous locomotor activity in adulthood. A deficit in acquisition of a specific learning task was observed in females only. The doses at which these findings were observed are at least 4 times the MRHD of 60 mg/day given to children on a mg/m2 basis.
In a study conducted in young rats, methylphenidate was administered orally at doses of up to 100 mg/kg/day for 9 weeks, starting early in the postnatal period (postnatal Day 7) and continuing through sexual maturity (postnatal Week 10). When these animals were tested as adults (postnatal Weeks 13 to 14), decreased spontaneous locomotor activity was observed in males and females previously treated with 50 mg/kg/day (approximately 4 times the MRHD of 60 mg/day given to children on a mg/m2 basis) or greater, and a deficit in the acquisition of a specific learning task was seen in females exposed to the highest dose (8 times the MRHD given to children on a mg/m2 basis). The no effect level for juvenile neurobehavioral development in rats was 5 mg/kg/day (approximately 0.5 times the MRHD given to children on a mg/m2 basis). The clinical significance of the long-term behavioral effects observed in rats is unknown.
8.5 Geriatric Use
Methylphenidate hydrochloride extended-release capsules have not been studied in the geriatric population.
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Boxed Warning 10/2023
Dosage and Administration (2.1) 10/2023
Warnings and Precautions (5.1, 5.2, 5.8, 5.9, 5.10) 10/2023
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
•
10 mg extended-release capsules white/light brown, (imprinted "NVR R10")
•
20 mg extended-release capsules white, (imprinted "NVR R20")
•
30 mg extended-release capsules yellow, (imprinted "NVR R30")
•
40 mg extended-release capsules light brown, (imprinted "NVR R40")
Extended-release capsules: 10 mg, 20 mg, 30 mg, and 40 mg (3).
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Pretreatment Screening
Prior to treating patients with methylphenidate hydrochloride extended-release capsules, assess:
•
for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see Warnings and Precautions (5.2)].
•
the family history and clinically evaluate patients for motor or verbal tics or Tourette’s syndrome before initiating methylphenidate hydrochloride extended-release capsules [see Warnings and Precautions (5.10)].
2.2 General Dosing Information
The recommended starting dose for methylphenidate hydrochloride extended- release capsules is 20 mg once daily. Increase dosage gradually, in increments of 10 mg weekly. Daily dosage above 60 mg is not recommended. When a lower initial dose is appropriate, patients may begin treatment with 10 mg.
Administer methylphenidate hydrochloride extended-release capsules orally once daily in the morning. Methylphenidate hydrochloride extended-release capsules may be swallowed as whole capsules or may be administered by sprinkling the capsule contents on a small amount of applesauce (see specific instructions below). Methylphenidate hydrochloride extended-release capsules and/or their contents should not be crushed, chewed, or divided.
The capsules may be carefully opened and the beads sprinkled over a spoonful of applesauce. The applesauce should not be warm because it could affect the modified release properties of this formulation. The mixture of drug and applesauce should be consumed immediately in its entirety. The drug and applesauce mixture should not be stored for future use.
2.3 Patients Currently Using Methylphenidate Hydrochloride Tablets
The recommended dose of methylphenidate hydrochloride extended-release capsules for patients currently taking methylphenidate hydrochloride tablets twice daily is provided below in Table 1.
Table 1: Recommended Dose Conversion from Methylphenidate Hydrochloride Tablets|
Previous Methylphenidate Hydrochloride Tablets’ Dose |
Recommended Methylphenidate Hydrochloride Extended-Release Capsules’ Dose |
|
5 mg methylphenidate hydrochloride tablets twice daily |
10 mg once daily |
|
10 mg methylphenidate hydrochloride tablets twice daily |
20 mg once daily |
|
15 mg methylphenidate hydrochloride tablets twice daily |
30 mg once daily |
|
20 mg methylphenidate hydrochloride tablets twice daily |
40 mg once daily |
|
30 mg methylphenidate hydrochloride tablets twice daily |
60 mg once daily |
2.4 Switching from Other Methylphenidate Products
If switching from other methylphenidate products, discontinue that treatment, and titrate with methylphenidate hydrochloride extended-release capsules using the titration schedule.
Do not substitute for other methylphenidate products on a milligram-per- milligram basis, because different methylphenidate base compositions and differing pharmacokinetic profiles [see Description (11), Clinical Pharmacology (12.3)].
Clinical judgment should be used when selecting the starting dose. Daily dosage above 60 mg is not recommended.
2.5 Dosage Reduction and Discontinuation
If paradoxical worsening of symptoms or other adverse reactions occur, reduce the dosage, or, if necessary, discontinue methylphenidate hydrochloride extended-release capsules. If improvement is not observed after appropriate dosage adjustment over a one-month period, the drug should be discontinued.
•
Administer orally once daily in the morning (2.2).
•
Capsules may be swallowed whole or opened and the entire contents sprinkled on applesauce (2.2).
•
Should not be crushed, chewed, or divided (2.2).
•
Patients new to methylphenidate: Start at 20 mg daily, titrating the dose weekly in 10-mg increments. Doses above 60 mg daily are not recommended (2.3).
•
For patients currently using methylphenidate hydrochloride tablets: Dosage is based on current dose regimen (2.4).
•
If switching from other methylphenidate products, discontinue treatment and titrate with methylphenidate hydrochloride extended release capsules (2.4).
OVERDOSAGE SECTION
10 OVERDOSAGE
Clinical Effects of Overdose
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
•
Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial
infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
•
CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures,
cerebral vascular accidents, and coma may occur.
•
Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
Overdose Management
Consider the possibility of multiple drug ingestion. The pharmacokinetic profile of methylphenidate hydrochloride extended-release capsules should be considered when treating patients with overdose. Because methylphenidate has a large volume of distribution and is rapidly metabolized, dialysis is not useful. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
DESCRIPTION SECTION
11 DESCRIPTION
Methylphenidate hydrochloride extended-release capsules are CNS stimulant.
Methylphenidate hydrochloride extended-release capsules is an extended-release formulation of methylphenidate for oral administration with a bi-modal release profile. Each bead-filled methylphenidate hydrochloride extended-release capsule contains half the dose as immediate-release beads and half as enteric- coated beads, thus providing an immediate release of methylphenidate and a second delayed release of methylphenidate.
The active substance in methylphenidate hydrochloride extended-release capsules is methyl α-phenyl-2-piperidineacetate hydrochloride, and its structural formula is

Methylphenidate hydrochloride USP is a white, odorless, fine crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77 g/mol.
Inactive ingredients: ammonio methacrylate copolymer, black iron oxide (10 and 40 mg capsules only), gelatin, methacrylic acid copolymer, polyethylene glycol, red iron oxide (10 and 40 mg capsules only), sugar spheres, talc, titanium dioxide, triethyl citrate, and yellow iron oxide (10, 30, and 40 mg capsules only).
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
•
10 mg extended-release capsules (NDC 0781-2361-01) white/light brown, (imprinted “NVR R10”) supplied in bottles of 100
•
20 mg extended-release capsules (NDC 0781-2362-01) white, (imprinted “NVR R20”) supplied in bottles of 100
•
30 mg extended-release capsules (NDC 0781-2363-01) yellow, (imprinted “NVR R30”) supplied in bottles of 100
•
40 mg extended-release capsules (NDC 0781-2364-01) light brown, (imprinted “NVR R40”) supplied in bottles of 100
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [See USP controlled room temperature].
Dispense in tight container (USP).
SPL MEDGUIDE SECTION
|
MEDICATION GUIDE |
|
What is the most important information I should know about methylphenidate hydrochloride extended-release capsules? Methylphenidate hydrochloride extended-release capsules may cause serious side effects, including: • o Your healthcare provider should check you or your child’s risk for abuse, misuse, and addiction before starting treatment with methylphenidate hydrochloride extended-release capsules and will monitor you or your child during treatment. o Methylphenidate hydrochloride extended-release capsules may lead to physical dependence after prolonged use, even if taken as directed by your healthcare provider. o Do not give methylphenidate hydrochloride extended-release capsules to anyone else. See “What are methylphenidate hydrochloride extended-release capsules?” for more information. o Keep methylphenidate hydrochloride extended-release capsules in a safe place and properly dispose of any unused medicine. See “How should I store methylphenidate hydrochloride extended-release capsules?” for more information. o Tell your healthcare provider if you or your child have ever abused or been dependent on alcohol, prescription medicines, or street drugs. • Your healthcare provider should check you or your child carefully for heart problems before starting methylphenidate hydrochloride extended-release capsules. Tell your healthcare provider if you or your child have any heart problems, heart disease, or heart defects. Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child has any signs of heart problems, such as chest pain, shortness of breath, or fainting while taking methylphenidate hydrochloride extended-release capsules. • Your healthcare provider should check you or your child’s blood pressure and heart rate regularly during treatment with methylphenidate hydrochloride extended-release capsules. • All Patients o new or worse behavior and thought problems o new or worse bipolar illness o new psychotic symptoms (such as hearing voices, believing things that are not true, are suspicious) or new manic symptoms Tell your healthcare provider about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression. Call your healthcare provider right away if you or your child have any new or worsening mental symptoms or problems while taking methylphenidate hydrochloride extended-release capsules, especially seeing or hearing things that are not real, believing things that are not real, or are suspicious. |
|
What are methylphenidate hydrochloride extended-release capsules? Methylphenidate hydrochloride extended-release capsules are a central nervous system (CNS) stimulant prescription medicine.**It is used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD).**Methylphenidate hydrochloride extended-release capsulesmay help increase attention and decrease impulsiveness and hyperactivity in patients with ADHD. Methylphenidate hydrochloride extended-release capsules should be used as a part of a total treatment program for ADHD that may include counseling or other therapies. It is not known if methylphenidate hydrochloride extended-release capsules are safe and effective in children under 6 years of age. **Methylphenidate hydrochloride extended-release capsules are a federally controlled substance (CII) because it contains methylphenidate that can be a target for people who abuse prescription medicines or street drugs.**Keep methylphenidate hydrochloride extended-release capsules in a safe place to protect it from theft. Never give your methylphenidate hydrochloride extended- release capsules to anyone else because it may cause death or harm them. Selling or giving away methylphenidate hydrochloride extended-release capsules may harm others and is against the law. |
|
Who should not take methylphenidate hydrochloride extended-release capsules? Methylphenidate hydrochloride extended-release capsules should not be taken if you or your child: • • |
|
Methylphenidate hydrochloride extended-release capsules may not be right for you or your child. Before starting methylphenidate hydrochloride extended- release capsules, tell your or your child’s healthcare provider about all health conditions (or a family history of), including: • • • • • • o • **Tell your healthcare provider about all of the medicines that you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.**Methylphenidate hydrochloride extended-release capsules and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking methylphenidate hydrochloride extended-release capsules. Your healthcare provider will decide whether methylphenidate hydrochloride extended-release capsules can be taken with other medicines. Especially tell your healthcare provider if you or your child takes: • • • Know the medicines that you or your child takes. Keep a list of your medicines with you to show your healthcare provider and pharmacist. • Do not start any new medicine while taking methylphenidate hydrochloride extended-release capsules without talking to your healthcare provider first. |
|
How should methylphenidate hydrochloride extended-release capsules be taken? • • • • • Your healthcare provider may do regular checks of the blood, heart, and blood pressure while taking methylphenidate hydrochloride extended-release capsules. Children should have their height and weight checked often while taking methylphenidate hydrochloride extended-release capsules. If you or your child take too many methylphenidate hydrochloride extended-release capsules, call your healthcare provider or Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away. |
|
What are possible side effects of methylphenidate hydrochloride extended- release capsules? • • • o o Tell your healthcare provider if you or your child have numbness, pain, skin color change, or sensitivity to temperature in the fingers or toes. Call your healthcare provider right away if you have or your child has any signs of unexplained wounds appearing on fingers or toes while taking methylphenidate hydrochloride extended-release capsules. • • • • o o o o o o o o Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store methylphenidate hydrochloride extended-release capsules? • • • • |
|
General information about the safe and effective use of methylphenidate hydrochloride extended-release capsules Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about methylphenidate hydrochloride extended-release capsules that is written for healthcare professionals. Do not use methylphenidate hydrochloride extended-release capsules for a condition for which it was not prescribed. Do not give methylphenidate hydrochloride extended-release capsules to other people, even if they have the same symptoms. It may harm them and it is against the law. |
|
What are the ingredients in methylphenidate hydrochloride extended-release capsules? **Active ingredient:**methylphenidate HCl **Inactive ingredients:**ammonio methacrylate copolymer, black iron oxide (10 and 40 mg capsules only), gelatin, methacrylic acid copolymer, polyethylene glycol, red iron oxide (10 and 40 mg capsules only), sugar spheres, talc, titanium dioxide, triethyl citrate, and yellow iron oxide (10, 30, and 40 mg capsules only). Distributed by: Sandoz Inc. Princeton, NJ 08540 For more information, call 1-800-525-8747. |
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 02/2024 |
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Methylphenidate hydrochloride extended-release capsules are Schedule II controlled substance.
9.2 Abuse
Methylphenidate hydrochloride extended-release capsules have a high potential for abuse and misuse which can lead to the development of a substance use disorder, including addiction [see Warnings and Precautions (5.1)]. Methylphenidate hydrochloride extended-release capsules can be diverted for non-medical use into illicit channels or distribution.
Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
Misuse and abuse of methylphenidate hydrochloride extended-release capsules may cause increased heart rate, respiratory rate, or blood pressure; sweating; dilated pupils; hyperactivity; restlessness; insomnia; decreased appetite; loss of coordination; tremors; flushed skin; vomiting; and/or abdominal pain. Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed with CNS stimulants abuse and/or misuse. Misuse and abuse of CNS stimulants, including methylphenidate hydrochloride extended- release capsules, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
9.3 Dependence
Physical Dependence
Methylphenidate hydrochloride extended-release capsules may produce physical dependence. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug.
Withdrawal signs and symptoms after abrupt discontinuation or dose reduction following prolonged use of CNS stimulants including methylphenidate hydrochloride extended-release capsules include dysphoric mood; depression; fatigue; vivid, unpleasant dreams; insomnia or hypersomnia; increased appetite; and psychomotor retardation or agitation.
Tolerance
Methylphenidate hydrochloride extended-release capsules may produce tolerance. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose).
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Methylphenidate hydrochloride is a CNS stimulant. The mode of therapeutic action in ADHD is not known.
12.2 Pharmacodynamics
Methylphenidate is a racemic mixture comprised of the d- and l-threo enantiomers. The d-threo enantiomer is more pharmacologically active than the l-threo enantiomer. Methylphenidate blocks the reuptake of norepinephrine and dopamine into the presynaptic neuron and increases the release of these monoamines into the extraneuronal space.
Cardiac Electrophysiology
A formal QT study has not been conducted in patients taking methylphenidate hydrochloride extended-release capsules.
The effect of dexmethylphenidate, the pharmacologically active d-enantiomer of methylphenidate hydrochloride tablet, on the QT interval was evaluated in a double-blind, placebo- and open-label active (moxifloxacin)-controlled study following single doses of dexmethylphenidate XR 40 mg (maximum recommended adult total daily dosage) in 75 healthy volunteers. Electrocardiograms were collected up to 12 hours postdose. Frederica’s method for heart rate correction was employed to derive the corrected QT interval (QTcF). The maximum mean prolongation of QTcF intervals was less than 5 ms, and the upper limit of the 90% confidence interval was below 10 ms for all time matched comparisons versus placebo. This was below the threshold of clinical concern and there was no evident-exposure response relationship.
12.3 Pharmacokinetics
Methylphenidate hydrochloride extended-release capsule produces a bi-modal plasma concentration-time profile (i.e., 2 distinct peaks approximately 4 hours apart) when administered orally to children diagnosed with ADHD and healthy adults.
No accumulation of methylphenidate is expected following multiple once daily oral dosing with methylphenidate hydrochloride extended-release capsules, however, there is a slight upward trend in the methylphenidate area under the curve and peak plasma concentrations (Cmax1 and Cmax2) after oral administration of methylphenidate hydrochloride extended-release 20 mg and 40 mg capsules to adults.
Absorption
The absolute oral bioavailability of methylphenidate in children was 22% ± 8% for d-methylphenidate and 5% ± 3% for l-methylphenidate. The relative bioavailability of methylphenidate hydrochloride extended-release capsules given once daily is comparable to the same total dose of methylphenidate hydrochloride tablets given in 2 doses 4 hours apart in both children and adults.
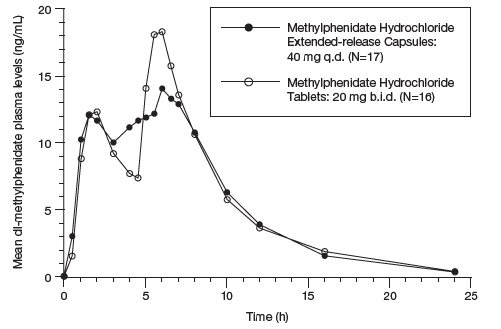
The initial rate of absorption for methylphenidate hydrochloride extended- release capsules is similar to that of methylphenidate hydrochloride tablets as shown by the similar rate parameters between the 2 formulations, i.e., initial lag time (Tlag), first peak concentration (Cmax1), and time to the first peak (Tmax1), which is reached in 1 to 3 hours. The mean time to the interpeak minimum (Tminip), and time to the second peak (Tmax2) are also similar for methylphenidate hydrochloride extended-release capsules given once daily and methylphenidate hydrochloride tablets given in 2 doses 4 hours apart (see Figure 1 and Table 1), although the ranges observed are greater for methylphenidate hydrochloride extended-release capsules.
Methylphenidate hydrochloride extended-release capsules given once daily exhibits a lower second peak concentration (Cmax2), higher interpeak minimum concentrations (Cminip), and less peak and trough fluctuations than methylphenidate hydrochloride tablets given in 2 doses given 4 hours apart. This is due to an earlier onset and more prolonged absorption from the delayed-release beads (see Figure 1 and Table 4).
Figure 1: Mean Plasma Concentration Time-profile of Methylphenidate After a Single Dose of Methylphenidate Hydrochloride Extended-Release Capsule 40 mg and Methylphenidate Hydrochloride Tablets 20 mg Given in Two Doses 4 Hours Apart
|
aN = 15. | ||||
|
Population |
Children |
Adult Males | ||
|
Methylphenidate Hydrochloride Tablets |
Methylphenidate Hydrochloride Extended-Release Capsules |
Methylphenidate Hydrochloride Tablets 10 mg & 10 mg |
Methylphenidate Hydrochloride Extended-Release Capsules |
|
N |
21 |
18 |
9 |
8 |
|
0.24 ± 0.44 |
0.28 ± 0.46 |
1.0 ± 0.5 |
0.7 ± 0.2 |
|
0 - 1 |
0 - 1 |
0.7 - 1.3 |
0.3 - 1.0 | |
|
1.8 ± 0.6 |
2.0 ± 0.8 |
1.9 ± 0.4 |
2.0 ± 0.9 |
|
1 - 3 |
1 - 3 |
1.3 - 2.7 |
1.3 - 4.0 | |
|
10.2 ± 4.2 |
10.3 ± 5.1 |
4.3 ± 2.3 |
5.3 ± 0.9 |
|
4.2 - 20.2 |
5.5 - 26.6 |
1.8 - 7.5 |
3.8 - 6.9 | |
|
4.0 ± 0.2 |
4.5 ± 1.2 |
3.8 ± 0.4 |
3.6 ± 0.6 |
|
4 - 5 |
2 - 6 |
3.3 - 4.3 |
2.7 - 4.3 | |
|
5.8 ± 2.7 |
6.1 ± 4.1 |
1.2 ± 1.4 |
3.0 ± 0.8 |
|
3.1 - 14.4 |
2.9 - 21.0 |
0.0 - 3.7 |
1.7 - 4.0 | |
|
5.6 ± 0.7 |
6.6 ± 1.5 |
5.9 ± 0.5 |
5.5 ± 0.8 |
|
5 - 8 |
5 - 11 |
5.0 - 6.5 |
4.3 - 6.5 | |
|
15.3 ± 7.0 |
10.2 ± 5.9 |
5.3 ± 1.4 |
6.2 ± 1.6 |
|
6.2 - 32.8 |
4.5 - 31.1 |
3.6 - 7.2 |
3.9 - 8.3 | |
|
AUC(0-∞) (ng/mL x h-1) |
102.4 ± 54.6 |
86.6 ± 64.0a |
37.8 ± 21.9 |
45.8 ± 10.0 |
|
40.5 - 261.6 |
43.3 - 301.44 |
14.3 - 85.3 |
34.0 - 61.6 | |
|
2.5 ± 0.8 |
2.4 ± 0.7a |
3.5 ± 1.9 |
3.3 ± 0.4 |
|
1.8 - 5.3 |
1.5 - 4.0 |
1.3 - 7.7 |
3.0 - 4.2 |
Effect of Food
Administration times relative to meals and meal composition may need to be individually titrated.
When methylphenidate hydrochloride extended-release capsule was administered with a high fat breakfast to adults, methylphenidate hydrochloride extended- release capsule had a longer lag time until absorption began and variable delays in the time until the first peak concentration, the time until the interpeak minimum, and the time until the second peak. The first peak concentration and the extent of absorption were unchanged after food relative to the fasting state, although the second peak was approximately 25% lower. The effect of a high fat lunch was not examined.
There were no differences in the pharmacokinetics of methylphenidate hydrochloride extended-release capsule when administered with applesauce, compared to administration in the fasting condition. There is no evidence of dose dumping in the presence or absence of food.
Effect of Alcohol
An in vitro study was conducted to explore the effect of alcohol on the release characteristics of methylphenidate from the methylphenidate hydrochloride extended-release 40 mg capsule dosage form. At an alcohol concentration of 40% there was a 98% release of methylphenidate in the first hour. The results with the 40 mg capsule are considered to be representative of the other available capsule strengths.
Distribution
Binding to plasma proteins is low (10% to 33%). The volume of distribution was 2.65 ± 1.11 L/kg for d-methylphenidate and 1.80 ± 0.91 L/kg for l-methylphenidate.
Elimination
The systemic clearance is 0.40 ± 0.12 L/h/kg for d-methylphenidate and 0.73 ± 0.28 L/h/kg for l-methylphenidate. In studies with methylphenidate hydrochloride extended-release capsules and methylphenidate hydrochloride tablets in adults, methylphenidate from methylphenidate hydrochloride tablets is eliminated from plasma with an average half-life of about 3.5 hours, (range, 1.3 to 7.7 hours). In children the average half-life is about 2.5 hours, with a range of about 1.5 to 5.0 hours. The rapid half-life in both children and adults may result in un-measurable concentrations between the morning and mid-day doses with methylphenidate hydrochloride tablets. No accumulation of methylphenidate is expected following multiple once a day oral dosing with methylphenidate hydrochloride extended-release capsules. The half- life of ritalinic acid is about 3 to 4 hours.
Metabolism
The absolute oral bioavailability of methylphenidate in children was 22% ± 8% for d-methylphenidate and 5% ± 3% for l-methylphenidate, suggesting pronounced presystemic metabolism. Biotransformation of methylphenidate by the carboxylesterase CES1A1 is rapid and extensive leading to the main, de- esterified metabolite α-phenyl-2-piperidine acetic acid (ritalinic acid), which has little or no pharmacologic activity. Only small amounts of hydroxylated metabolites (e.g., hydroxymethylphenidate and hydroxyritalinic acid) are detectable in plasma. Therapeutic activity is principally due to the parent compound.
Excretion
After oral administration of an immediate release formulation of methylphenidate, 78% to 97% of the dose is excreted in the urine and 1% to 3% in the feces in the form of metabolites within 48 to 96 hours. Only small quantities (less than 1%) of unchanged methylphenidate appear in the urine. Most of the dose is excreted in the urine as ritalinic acid (60% to 86%), the remainder being accounted for by minor metabolite.
Studies in Specific Populations
Male and Female Patients
There were no apparent gender differences in the pharmacokinetics of methylphenidate between healthy male and female adults when administered methylphenidate hydrochloride extended-release capsules.
Racial or Ethnic Groups
There is insufficient experience with the use of methylphenidate hydrochloride extended-release capsules to detect ethnic variations in pharmacokinetics.
Pediatric Patients
The pharmacokinetics of methylphenidate hydrochloride extended-release capsules was examined in 18 children with ADHD between 7 and 12 years of age. Fifteen of these children were between 10 and 12 years of age. The time until the between peak minimum, and the time until the second peak were delayed and more variable in children compared to adults. After a 20-mg dose of methylphenidate hydrochloride extended-release capsule, concentrations in children were approximately twice the concentrations observed in 18- to 35- year- old adults. This higher exposure is almost completely due to the smaller body size and total volume of distribution in children, as apparent clearance normalized to body weight is independent of age.
Patients with Renal Impairment
Methylphenidate hydrochloride extended-release capsules have not been studied in renally-impaired patients. Renal impairment is expected to have minimal effect on the pharmacokinetics of methylphenidate since less than 1% of a radiolabeled dose is excreted in the urine as unchanged compound, and the major metabolite (ritalinic acid), has little or no pharmacologic activity.
Patients with Hepatic Impairment
Methylphenidate hydrochloride extended-release capsules have not been studied in patients with hepatic impairment. Hepatic impairment is expected to have minimal effect on the pharmacokinetics of methylphenidate since it is metabolized primarily to ritalinic acid by nonmicrosomal hydrolytic esterases that are widely distributed throughout the body.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
In a lifetime carcinogenicity study carried out in B6C3F1 mice, methylphenidate caused an increase in hepatocellular adenomas, and in males only, an increase in hepatoblastomas at a daily dose of approximately 60 mg/kg/day. This dose is approximately 2 times the MRHD of 60 mg/day given to children on a mg/m2 basis. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors, and the significance of these results to humans is unknown.
Methylphenidate did not cause any increase in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day, which is approximately 4 times the MRHD (children) on a mg/m2 basis.
In a 24-week carcinogenicity study in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. Male and female mice were fed diets containing the same concentration of methylphenidate as in the lifetime carcinogenicity study; the high-dose groups were exposed to 60 to 74 mg/kg/day of methylphenidate.
Mutagenesis
Methylphenidate was not mutagenic in the in vitro Ames reverse mutation assay, in the in vitro mouse lymphoma cell forward mutation assay, or in the in vitro chromosomal aberration assay using human lymphocytes. Sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response, in an in vitro assay in cultured Chinese Hamster Ovary cells. Methylphenidate was negative in vivo in males and females in the mouse bone marrow micronucleus assay.
Impairment of Fertility
Methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week continuous breeding study. The study was conducted at doses up to 160 mg/kg/day, approximately 10 times the maximum recommended dose of 60 mg/day given to adolescents on a mg/m2 basis.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Children and Adolescents
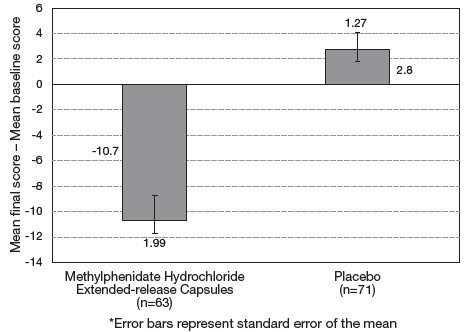
Methylphenidate hydrochloride extended-release capsules were evaluated in a randomized, double-blind, placebo-controlled, parallel group clinical study in which 134 children, ages 6 to 12 years, with DSM-IV diagnoses of ADHD received a single morning dose of methylphenidate hydrochloride extended-release capsules in the range of 10 to 40 mg/day, or placebo, for up to 2 weeks. The doses used were the optimal doses established in a previous individual dose titration phase. In that titration phase, 53 of 164 patients (32%) started on a daily dose of 10 mg and 111 of 164 patients (68%) started on a daily dose of 20 mg or higher. The patient’s regular schoolteacher completed the Conners ADHD/DSM-IV Scale for Teachers (CADS-T) at baseline and the end of each week. The CADS-T assesses symptoms of hyperactivity and inattention. The change from baseline of the (CADS-T) scores during the last week of treatment was analyzed as the primary efficacy parameter. Patients treated with methylphenidate hydrochloride extended-release capsules showed a statistically significant improvement in symptom scores from baseline [Mean (final score - baseline) = -10.7 points] over patients who received placebo [Mean (final score - baseline) = +2.8 points]. The lower the final score on the CADS-T scale from baseline, the less severe the disease is. This demonstrates that a single morning dose of methylphenidate hydrochloride extended-release capsule exerts a treatment effect in ADHD.
Figure 2: CADS-T Total Subscale - Mean Change from Baseline*
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Abuse, Misuse, and Addiction
Educate patients and their families about the risks of abuse, misuse, and addiction of methylphenidate hydrochloride extended-release capsules, which can lead to overdose and death, and proper disposal of any unused drug [see Warnings and Precautions (5.1), Drug Abuse andDependence (9.2), Overdosage (10)]. Advise patients to store methylphenidate hydrochloride extended-release capsules in a safe place, preferably locked, and instruct patients to not give methylphenidate hydrochloride extended-release capsules to anyone else.
Risks to Patients with Serious Cardiac Disease
Advise patients that there are potential risks to patients with serious cardiac disease, including sudden death, with use of methylphenidate hydrochloride extended-release capsules. Instruct patients to contact a healthcare provider immediately if they develop symptoms, such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease [see Warnings andPrecautions (5.2)].
Increased Blood Pressure and Heart Rate
Instruct patients that methylphenidate hydrochloride extended-release capsules can cause elevations of their blood pressure and pulse rate [see Warnings and Precautions (5.3)].
Psychiatric Adverse Reactions
Advise patients that methylphenidate hydrochloride extended-release capsules, at recommended doses, can cause psychotic or manic symptoms, even in patients without prior history of psychotic symptoms or mania [see Warnings and Precautions (5.4)].
Priapism
Advise patients of the possibility of painful or prolonged penile erections (priapism). Instruct them to seek immediate medical attention in the event of priapism [see Warnings and Precautions (5.5)].
Circulation Problems in Fingers and Toes [Peripheral Vasculopathy, Including Raynaud’s Phenomenon]
Instruct patients beginning treatment with methylphenidate hydrochloride extended-release capsules about the risk of peripheral vasculopathy, including Raynaud’s phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red. Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking methylphenidate hydrochloride extended-release capsules. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients [see Warnings and Precautions (5.6)].
Long Term Suppression of Growth in Pediatric Patients
Advise patients that methylphenidate hydrochloride extended-release capsules may cause slowing of growth and weight loss [see Warnings and Precautions (5.7)].
Increased Intraocular Pressure (IOP) and Glaucoma
Advise patients that IOP and glaucoma may occur during treatment with methylphenidate hydrochloride extended-release capsules [see Warnings and Precautions (5.9)].
Motor and Verbal Tics and Worsening of Tourette’s Syndrome
Advise patients that motor and verbal tics and worsening of Tourette’s Syndrome may occur during treatment with
methylphenidate hydrochloride extended-release capsules. Instruct patients to notify their healthcare provider if emergence of new tics or worsening of tics or Tourette’s syndrome occurs [see Warnings and Precautions (5.10)].
Alcohol Effect
Advise patients to avoid alcohol while taking methylphenidate hydrochloride extended-release capsules. Consumption of alcohol while taking methylphenidate hydrochloride extended-release capsules may result in a more rapid release of the dose of methylphenidate [see Clinical Pharmacology (12.3)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in patients exposed to methylphenidate hydrochloride extended-release capsules during pregnancy [see Use in Specific Populations (8.1)].
Distributed by:
Sandoz Inc.
Princeton, NJ 08540
