CVS Gentle Laxative
CVS Pharmacy Gentle Laxatives 8 ct
9c6a651a-f062-4784-9d82-156f9f745223
HUMAN OTC DRUG LABEL
Jul 13, 2025
CVS Pharmacy
DUNS: 062312574
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Bisacodyl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Product Label
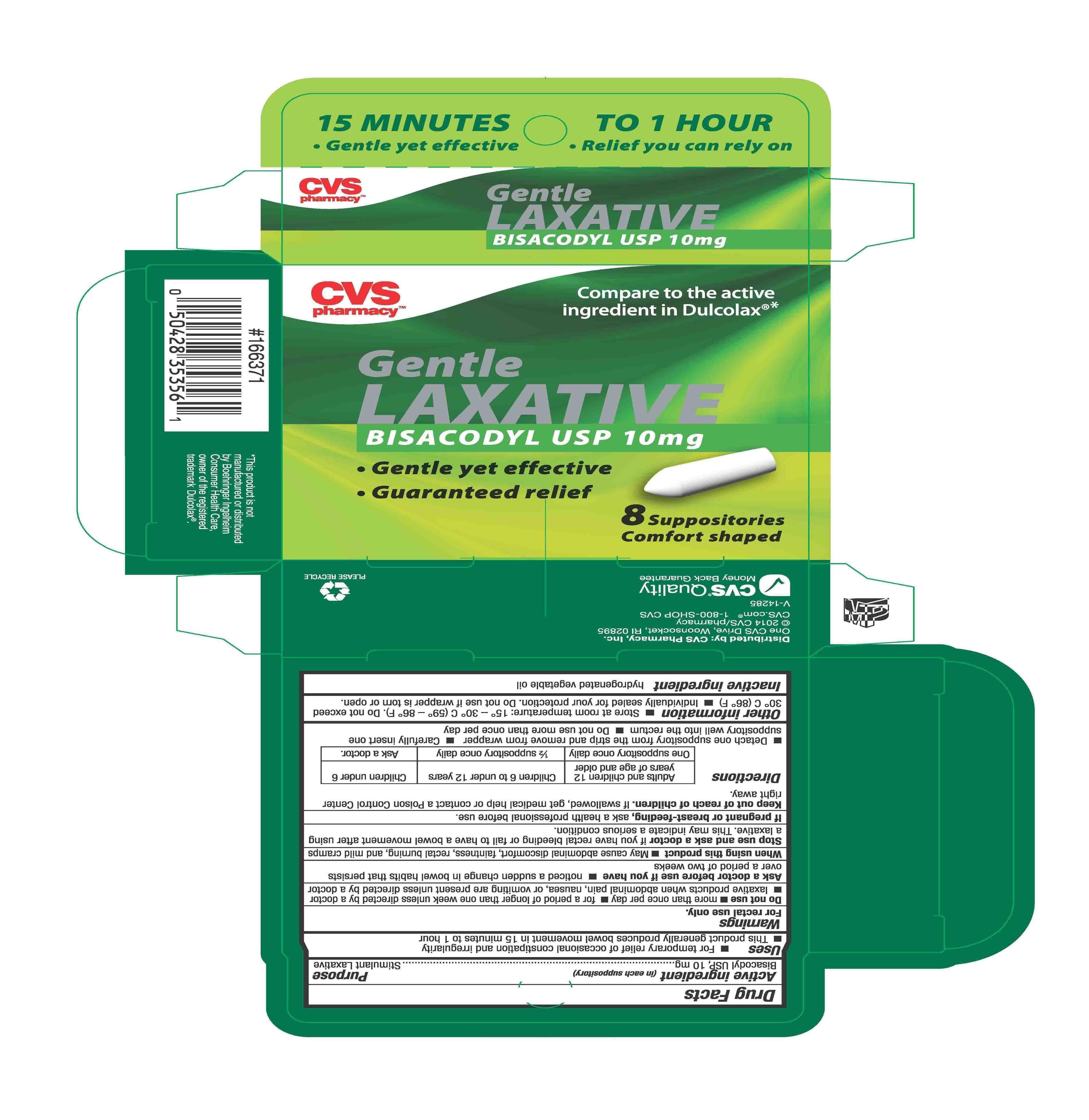
INDICATIONS & USAGE SECTION
Uses
Uses - For relief of occasional constipation and irregularity
-This product generally produces bowel movement in 15 minutes to 1 hour
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding
If pregnant or breast-feeding, ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
OTC - STOP USE SECTION
Stop use and ask a doctor
Stop use and ask a doctor if you have rectal bleeding or fail to have bowel movement after using a laxative. This may indicate a serious condition
OTC - DO NOT USE SECTION
Do not use
Do not use - more than once per day - for a period of longer than one week unless directed by a doctor - laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor
OTC - ASK DOCTOR SECTION
Ask a doctor
Ask a doctor before use if you have - noticed a sudden change in bowel habits that persists over a period of two weeks
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient (in each Suppository)
Purpose
Bisacodyl USP, 10 mg.................................Stimulant Laxative
WARNINGS SECTION
Warnings
For rectal use only.
OTC - WHEN USING SECTION
When using this product
When using this product - May cause abdominal discomfort, faintness, rectal burning, and mild cramps
DOSAGE & ADMINISTRATION SECTION
Directions
Adults and children 12 years of age and older Children 6 to under 12 years Children under 6
One suppository once daily 1/2 suppository once daily Ask doctor.
-Detach one suppository from the strip and remove from foil - Carefully insert one suppository well into the rectum
-Do not use more than once per day
STORAGE AND HANDLING SECTION
Other information
Other information - Store at room temperature: 15 o - 30 oC (59 o - 86 oF). Do not exceed 30 oC (86 oF) - Individually sealed for your protection. Do not use if foil is torn or open
INACTIVE INGREDIENT SECTION
Inactive ingredient
Inactive ingredient Hydrogenated vegetable oil
OTC - PURPOSE SECTION
Purpose
Stimulant Laxative
