BEIJIXI Liquid Wart Corn Callus Remover
3d4fb1c5-b96c-0630-e063-6294a90af35b
HUMAN OTC DRUG LABEL
Aug 26, 2025
TT Beauty Trading Co., Ltd.
DUNS: 661400516
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
GLYCERIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
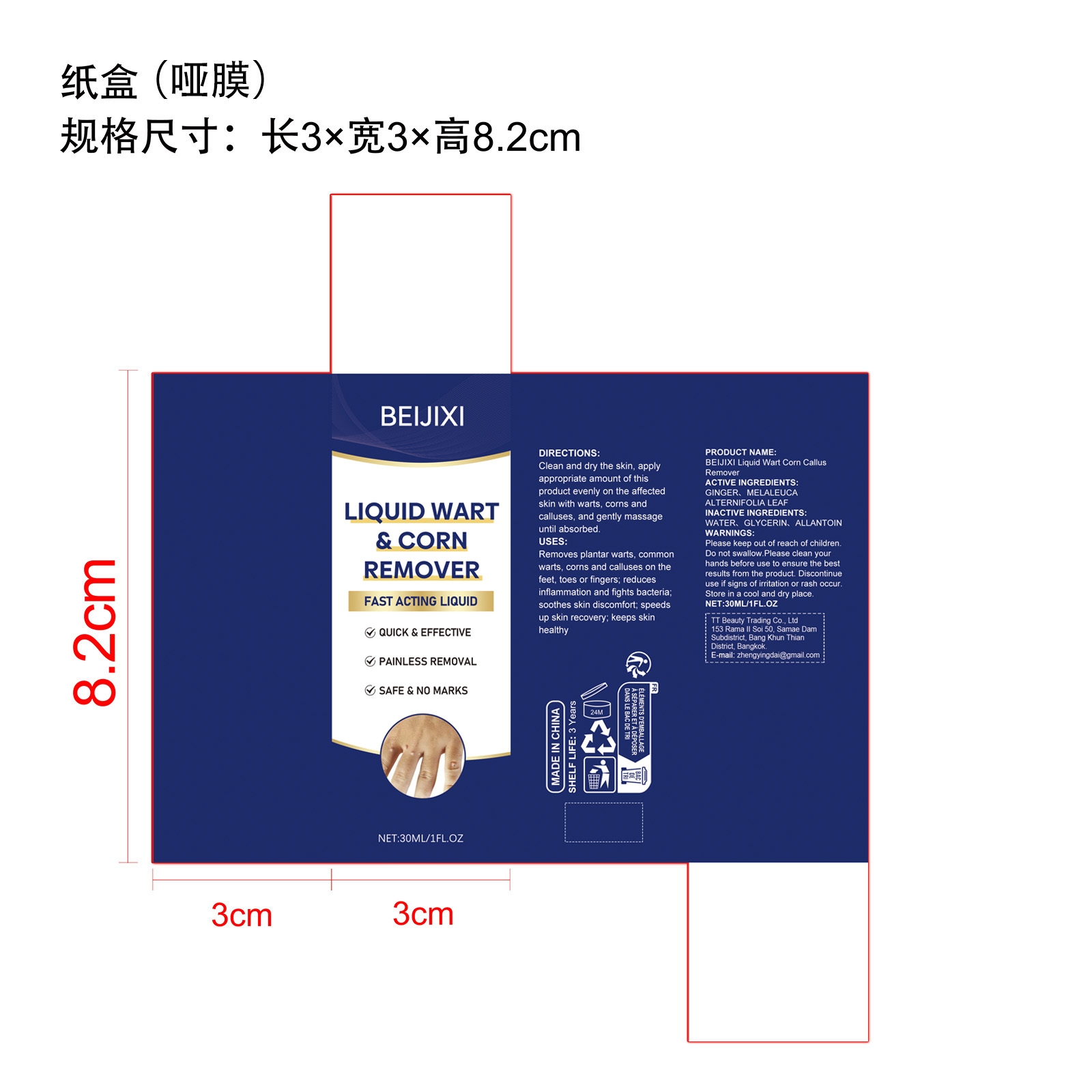
INDICATIONS & USAGE SECTION
Clean and dry the skin, apply appropriate amount of this product evenly on the affected skin with warts, corns and calluses, and gently massage until absorbed.
DOSAGE & ADMINISTRATION SECTION
Clean and dry the skin, apply appropriate amount of this product evenly on the affected skin with warts, corns and calluses, and gently massage until absorbed.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Please keep out of reach of children. Do not swallow.
STORAGE AND HANDLING SECTION
Store in a cool and dry place.
INACTIVE INGREDIENT SECTION
WATER. GLYCERIN, ALLANTOIN
OTC - STOP USE SECTION
Discontinue use if signs of irritation or rash occur.
OTC - ACTIVE INGREDIENT SECTION
GINGER、MELALEUCA ALTERNIFOLIA LEAF
OTC - WHEN USING SECTION
Clean and dry the skin, apply appropriate amount of this product evenly on the affected skin with warts, corns and calluses, and gently massage until absorbed.
OTC - PURPOSE SECTION
Removes plantar warts, common warts, corns and calluses on the feet, toes or fingers; reduces inflammation and fights bacteria; soothes skin discomfort; speeds up skin recovery; keeps skin healthy
WARNINGS SECTION
Please keep out of reach of children. Do not swallow.Please clean your hands before use to ensure the best results from the product. Discontinue use if signs of irritation or rash occur. Store in a cool and dry place.
OTC - DO NOT USE SECTION
Discontinue use if signs of irritation or rash occur.
