PEMETREXED
These highlights do not include all the information needed to use PEMETREXED INJECTION safely and effectively. See full prescribing information for PEMETREXED INJECTION. PEMETREXED INJECTION, for intravenous use Initial U.S. Approval: 2004
3e087418-69ff-47fa-93c2-f28699309433
HUMAN PRESCRIPTION DRUG LABEL
Feb 1, 2023
Hospira, Inc.
DUNS: 141588017
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
PEMETREXED
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
PEMETREXED
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
PEMETREXED
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
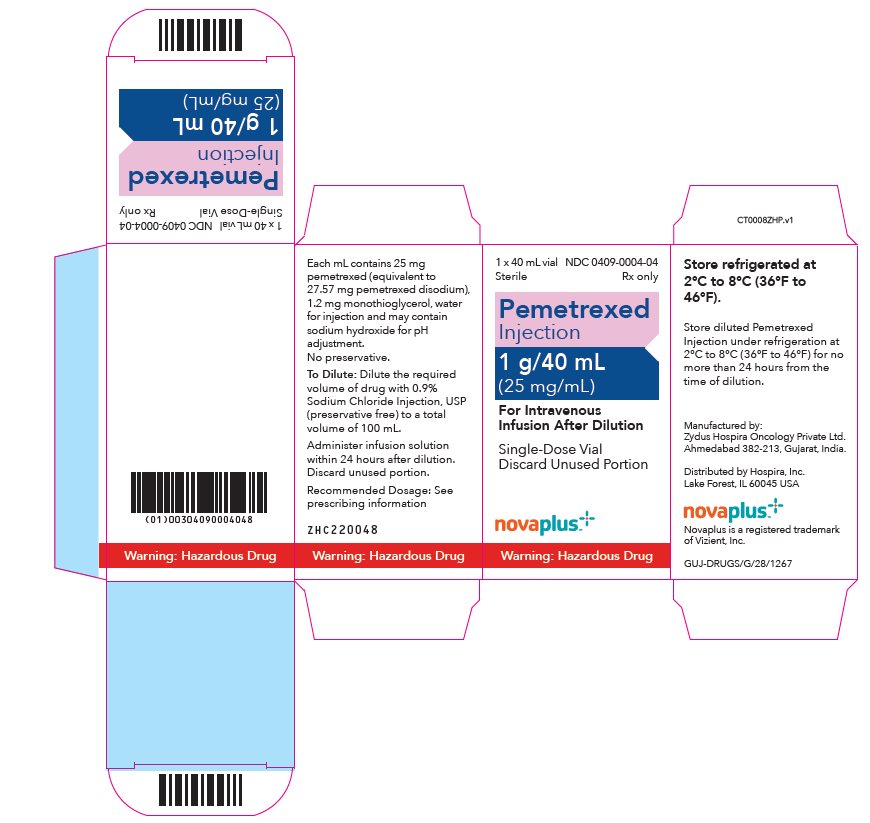
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 40 mL Vial Carton
1 x 40 mL vial
NDC 0409-0004-04
Sterile
Rx only
Pemetrexed
Injection
1 g/40 mL
(25 mg/mL)
For Intravenous
Infusion After Dilution
Single Dose Vial
Discard Unused Portion
Novaplus
Warning: Hazardous Drug
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Non-Squamous Non-Small Cell Lung Cancer (NSCLC)
Pemetrexed Injection is indicated:
•
In combination with cisplatin for the initial treatment of patients with locally advanced or metastatic, non-squamous, non-small cell lung cancer (NSCLC).
•
As a single agent for the maintenance treatment of patients with locally advanced or metastatic, non-squamous NSCLC whose disease has not progressed after four cycles of platinum-based first-line chemotherapy.
•
As a single agent for the treatment of patients with recurrent, metastatic non-squamous, NSCLC after prior chemotherapy.
Limitations of Use: Pemetrexed Injection is not indicated for the treatment of patients with squamous cell, non-small cell lung cancer [see Clinical Studies 14.1].
1.2 Mesothelioma
Pemetrexed Injection is indicated, in combination with cisplatin, for the initial treatment of patients with malignant pleural mesothelioma whose disease is unresectable or who are otherwise not candidates for curative surgery.
Pemetrexed Injection is a folate analog metabolic inhibitor indicated:
•
In combination with cisplatin for the initial treatment of patients with locally advanced or metastatic, non-squamous, non-small cell lung cancer (NSCLC). (1.1)
•
As a single agent for the maintenance treatment of patients with locally advanced or metastatic, non -squamous NSCLC whose disease has not progressed after four cycles of platinum-based first-line chemotherapy. (1.1)
•
As a single agent for the treatment of patients with recurrent, metastatic non-squamous, NSCLC after prior chemotherapy. (1.1)
•
Limitations of Use: Pemetrexed Injection is not indicated for the treatment of patients with squamous cell, non-small cell lung cancer. (1.1)
•
Initial treatment, in combination with cisplatin, of patients with malignant pleural mesothelioma whose disease is unresectable or who are otherwise not candidates for curative surgery. (1.2)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression and Increased Risk of Myelosuppression without Vitamin
Supplementation
Pemetrexed can cause severe myelosuppression resulting in a requirement for transfusions and which may lead to neutropenic infection. The risk of myelosuppression is increased in patients who do not receive vitamin supplementation. In Study JMCH, incidences of Grade 3–4 neutropenia (38% versus 23%), thrombocytopenia (9% versus 5%), febrile neutropenia (9% versus 0.6%), and neutropenic infection (6% versus 0) were higher in patients who received pemetrexed plus cisplatin without vitamin supplementation as compared to patients who were fully supplemented with folic acid and vitamin B12 prior to and throughout pemetrexed plus cisplatin treatment.
Initiate supplementation with oral folic acid and intramuscular vitamin B12 prior to the first dose of Pemetrexed Injection; continue vitamin supplementation during treatment and for 21 days after the last dose of Pemetrexed Injection to reduce the severity of hematologic and gastrointestinal toxicity of Pemetrexed Injection [see Dosage and Administration (2.4)]. Obtain a complete blood count at the beginning of each cycle. Do not administer Pemetrexed Injection until the ANC is at least 1500 cells/mm3 and platelet count is at least 100,000 cells/mm3. Permanently reduce Pemetrexed Injection in patients with an ANC of less than 500 cells/mm3 or platelet count of less than 50,000 cells/mm3 in previous cycles [see Dosage and Administration (2.6)].
In Studies JMDB and JMCH, among patients who received vitamin supplementation, incidence of Grade 3–4 neutropenia was 15% and 23%, the incidence of Grade 3–4 anemia was 6% and 4%, and incidence of Grade 3–4 thrombocytopenia was 4% and 5%, respectively. In Study JMCH, 18% of patients in the pemetrexed arm required red blood cell transfusions compared to 7% of patients in the cisplatin arm [see Adverse Reactions (6.1)]. In Studies JMEN, PARAMOUNT, and JMEI, where all patients received vitamin supplementation, incidence of Grade 3–4 neutropenia ranged from 3% to 5%, and incidence of Grade 3–4 anemia ranged from 3% to 5%.
5.2 Renal Failure
Pemetrexed can cause severe, and sometimes fatal, renal toxicity. The incidences of renal failure in clinical studies in which patients received pemetrexed with cisplatin were: 2.1% in Study JMDB and 2.2% in Study JMCH. The incidence of renal failure in clinical studies in which patients received pemetrexed as a single agent ranged from 0.4% to 0.6% (Studies JMEN, PARAMOUNT, and JMEI [see Adverse Reactions (6.1)]). Determine creatinine clearance before each dose and periodically monitor renal function during treatment with Pemetrexed Injection. Withhold Pemetrexed Injection in patients with a creatinine clearance of less than 45 mL/minute [see Dosage and Administration (2.3)].
5.3 Bullous and Exfoliative Skin Toxicity
Serious and sometimes fatal, bullous, blistering and exfoliative skin toxicity, including cases suggestive of Stevens-Johnson Syndrome/Toxic epidermal necrolysis can occur with pemetrexed. Permanently discontinue Pemetrexed Injection for severe and life-threatening bullous, blistering or exfoliating skin toxicity.
5.4 Interstitial Pneumonitis
Serious interstitial pneumonitis, including fatal cases, can occur with pemetrexed treatment. Withhold Pemetrexed Injection for acute onset of new or progressive unexplained pulmonary symptoms such as dyspnea, cough, or fever pending diagnostic evaluation. If pneumonitis is confirmed, permanently discontinue Pemetrexed Injection.
5.5 Radiation Recall
Radiation recall can occur with pemetrexed in patients who have received radiation weeks to years previously. Monitor patients for inflammation or blistering in areas of previous radiation treatment. Permanently discontinue Pemetrexed Injection for signs of radiation recall.
5.6 Increased Risk of Toxicity with Ibuprofen in Patients with Renal
Impairment
Exposure to pemetrexed is increased in patients with mild to moderate renal impairment who take concomitant ibuprofen, increasing the risks of adverse reactions of pemetrexed. In patients with creatinine clearances between 45 mL/min and 79 mL/min, avoid administration of ibuprofen for 2 days before, the day of, and 2 days following administration of Pemetrexed Injection. If concomitant ibuprofen use cannot be avoided, monitor patients more frequently for Pemetrexed Injection adverse reactions, including myelosuppression, renal, and gastrointestinal toxicity [see Dosage and Administration (2.5), Drug Interactions (7), and Clinical Pharmacology (12.3)].
5.7 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, Pemetrexed Injection can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, intravenous administration of pemetrexed to pregnant mice during the period of organogenesis was teratogenic, resulting in developmental delays and increased malformations at doses lower than the recommended human dose of 500 mg/m2. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Pemetrexed Injection and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Pemetrexed Injection and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
•
Myelosuppression: Can cause severe bone marrow suppression resulting in cytopenia and an increased risk of infection. Do not administer Pemetrexed Injection when the absolute neutrophil count is less than 1500 cells/mm3 and platelets are less than 100,000 cells/mm3. Initiate supplementation with oral folic acid and intramuscular vitamin B12 to reduce the severity of hematologic and gastrointestinal toxicity of Pemetrexed Injection. (2.4, 5.1)
•
Renal Failure: Can cause severe, and sometimes fatal, renal failure. Do not administer when creatinine clearance is less than 45 mL/min. (2.3, 5.2)
•
Bullous and Exfoliative Skin Toxicity: Permanently discontinue for severe and life-threatening bullous, blistering or exfoliating skin toxicity. (5.3)
•
Interstitial Pneumonitis: Withhold for acute onset of new or progressive unexplained pulmonary symptoms. Permanently discontinue if pneumonitis is confirmed. (5.4)
•
Radiation Recall: Can occur in patients who received radiation weeks to years previously; permanently discontinue for signs of radiation recall. (5.5)
•
Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of the potential risk to a fetus and to use effective contraception. (5.7, 8.1, 8.3)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Effects of Ibuprofen on Pemetrexed
Ibuprofen increases exposure (AUC) of pemetrexed [see Clinical Pharmacology (12.3)]. In patients with creatinine clearance between 45 mL/min and 79 mL/min:
•
Avoid administration of ibuprofen for 2 days before, the day of, and 2 days following administration of Pemetrexed Injection [see Dosage and Administration (2.5)].
•
Monitor patients more frequently for myelosuppression, renal, and gastrointestinal toxicity, if concomitant administration of ibuprofen cannot be avoided.
Ibuprofen increased risk of Pemetrexed Injection toxicity in patients with mild to moderate renal impairment. Modify the ibuprofen dosage as recommended for patients with a creatinine clearance between 45 mL/min and 79 mL/min. (2.5, 5.6, 7)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Non-Squamous NSCLC
•
The recommended dose of Pemetrexed Injection when administered with cisplatin for initial treatment of locally advanced or metastatic non-squamous NSCLC in patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater is 500 mg/m2 as an intravenous infusion over 10 minutes administered prior to cisplatin on Day 1 of each 21-day cycle for up to six cycles in the absence of disease progression or unacceptable toxicity.
•
The recommended dose of Pemetrexed Injection for maintenance treatment of non-squamous NSCLC in patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater is 500 mg/m2 as an intravenous infusion over 10 minutes on Day 1 of each 21-day cycle until disease progression or unacceptable toxicity after four cycles of platinum-based first-line chemotherapy.
•
The recommended dose of Pemetrexed Injection for treatment of recurrent non-squamous NSCLC in patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater is 500 mg/m2 as an intravenous infusion over 10 minutes on Day 1 of each 21-day cycle until disease progression or unacceptable toxicity.
2.2 Recommended Dosage for Mesothelioma
The recommended dose of Pemetrexed Injection when administered with cisplatin in patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater is 500 mg/m2 as an intravenous infusion over 10 minutes on Day 1 of each 21-day cycle until disease progression or unacceptable toxicity.
2.3 Renal Impairment
Pemetrexed Injection dosing recommendations are provided for patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater [see Dosage and Administration (2.1, 2.2)]. There is no recommended dose for patients whose creatinine clearance is less than 45 mL/min [see Use in Specific Populations (8.6)].
2.4 Premedication and Concomitant Medications to Mitigate Toxicity
Vitamin Supplementation
•
Initiate folic acid 400 mcg to 1000 mcg orally once daily, beginning 7 days before the first dose of Pemetrexed Injection and continuing until 21 days after the last dose of Pemetrexed Injection [see Warnings and Precautions (5.1)].
•
Administer vitamin B12, 1 mg intramuscularly, 1 week prior to the first dose of Pemetrexed Injection and every 3 cycles thereafter. Subsequent vitamin B12 injections may be given the same day as treatment with Pemetrexed Injection [see Warnings and Precautions (5.1)].**Do not substitute oral vitamin B****12**** for intramuscular vitamin B****12**.
Corticosteroids
•
Administer dexamethasone 4 mg orally twice daily for three consecutive days, beginning the day before each Pemetrexed Injection administration.
2.5 Dosage Modification of Ibuprofen in Patients with Mild to Moderate
Renal Impairment Receiving Pemetrexed Injection
In patients with creatinine clearances between 45 mL/min and 79 mL/min, modify administration of ibuprofen as follows [see Warnings and Precautions (5.6), Drug Interactions (7) and Clinical Pharmacology (12.3)]:
•
Avoid administration of ibuprofen for 2 days before, the day of, and 2 days following administration of Pemetrexed Injection.
•
Monitor patients more frequently for myelosuppression, renal, and gastrointestinal toxicity, if concomitant administration of ibuprofen cannot be avoided.
2.6 Dosage Modifications for Adverse Reactions
Obtain complete blood count on Days 1, 8, and 15 of each cycle. Assess creatinine clearance prior to each cycle. Do not administer Pemetrexed Injection if the creatinine clearance is less than 45 mL/min.
Delay initiation of the next cycle of Pemetrexed Injection until:
•
Recovery of non-hematologic toxicity to Grade 0–2,
•
Absolute neutrophil count (ANC) is 1500 cells/mm3 or higher, and
•
Platelet count is 100,000 cells/mm3 or higher.
Upon recovery, modify the dosage of Pemetrexed Injection in the next cycle as specified in Table 1.
For dosing modifications for cisplatin, refer to the prescribing information for cisplatin.
Table 1: Recommended Dosage Modifications for Adverse Reactions*|
Toxicity in Most Recent Treatment Cycle |
Pemetrexed Injection Dose Modification for Next Cycle |
|---|---|
| |
|
Myelosuppressive toxicity [see Warnings and Precautions (5.1)] | |
|
ANC less than 500/mm3and platelets greater than or equal to 50,000/mm3 |
75% of previous dose |
|
Platelet count less than 50,000/mm3 with bleeding |
50% of previous dose |
|
Recurrent Grade 3 or 4 myelosuppression after 2 dose reductions |
Discontinue |
|
Non-hematologic toxicity | |
|
Any Grade 3 or 4 toxicities EXCEPT mucositis or neurologic toxicity or diarrhea requiring hospitalization |
75% of previous dose |
|
Grade 3 or 4 mucositis |
50% of previous dose |
|
Renal toxicity [see Warnings and Precautions (5.2)] |
Withhold until creatinine clearance is 45 mL/min or greater |
|
Grade 3 or 4 neurologic toxicity |
Permanently discontinue |
|
Recurrent Grade 3 or 4 non-hematologic toxicity after 2 dose reductions |
Permanently discontinue |
|
Severe and life-threatening Skin Toxicity [see Warnings and Precautions (5.3)] |
Permanently discontinue |
|
Interstitial Pneumonitis [see Warnings and Precautions (5.4)] |
Permanently discontinue |
2.7 Preparation for Administration
Pemetrexed Injection is a hazardous drug. Follow applicable special handling and disposal procedures.1
•
Calculate the dose of Pemetrexed Injection and determine the number of vials needed.
•
Withdraw the calculated dose of Pemetrexed Injection from the vial(s) and discard vial with any unused portion.
•
Dilute Pemetrexed Injection with 0.9% Sodium Chloride Injection, USP (preservative-free) to achieve a total volume of 100 mL for intravenous infusion.
•
Visually inspect for particulate matter and discoloration prior to administration. Discard if particulate matter or discoloration is observed.
•
Administer Pemetrexed Injection as an intravenous infusion over 10 minutes.
•
If not used immediately, store diluted product under refrigerated conditions [2°C to 8°C (36°F to 46°F)] for no more than 24 hours from the time of dilution. Discard after 24 hours.
•
The recommended dose of Pemetrexed Injection, administered as a single agent or with cisplatin, in patients with creatinine clearance of 45 mL/minute or greater is 500 mg/m2 as an intravenous infusion over 10 minutes on Day 1 of each 21-day cycle. (2.1, 2.2)
•
Initiate folic acid 400 mcg to 1000 mcg orally, once daily, beginning 7 days prior to the first dose of Pemetrexed Injection and continue until 21 days after the last dose of Pemetrexed Injection. (2.4)
•
Administer vitamin B12, 1 mg intramuscularly, 1 week prior to the first dose of Pemetrexed Injection and every 3 cycles. (2.4)
•
Administer dexamethasone 4 mg orally, twice daily the day before, the day of, and the day after Pemetrexed Injection administration. (2.4)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been conducted with pemetrexed. Pemetrexed was clastogenic in an in vivo micronucleus assay in mouse bone marrow but was not mutagenic in multiple in vitro tests (Ames assay, Chinese Hamster Ovary cell assay).
Pemetrexed administered intraperitoneally at doses of ≥0.1 mg/kg/day to male mice (approximately 0.0006 times the recommended human dose based on BSA) resulted in reduced fertility, hypospermia, and testicular atrophy.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Pemetrexed Injection is a clear, colorless to pale yellow ready-to-dilute solution in a single-dose vial for intravenous infusion.
NDC 0409-0020-02: Carton containing one (1) single-dose vial, 100 mg/4 mL (25 mg/mL).
NDC 0409-0021-03: Carton containing one (1) single-dose vial, 500 mg/20 mL (25 mg/mL).
NDC 0409-0004-04: Carton containing one (1) single-dose vial, 1 g/40 mL (25 mg/mL).
Storage and Handling
Store Pemetrexed Injection at controlled refrigerated temperature, 2°C to 8°C (36°F to 46°F).
Pemetrexed Injection is a hazardous drug. Follow applicable special handling and disposal procedures. [see References (15)]
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Premedication and Concomitant Medication: Instruct patients to take folic acid as directed and to keep appointments for vitamin B12 injections to reduce the risk of treatment-related toxicity. Instruct patients of the requirement to take corticosteroids to reduce the risks of treatment-related toxicity [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
Myelosuppression: Inform patients of the risk of low blood cell counts and instruct them to immediately contact their physician for signs of infection, fever, bleeding, or symptoms of anemia [see Warnings and Precautions (5.1)].
Renal Failure: Inform patients of the risks of renal failure, which may be exacerbated in patients with dehydration arising from severe vomiting or diarrhea. Instruct patients to immediately contact their healthcare provider for a decrease in urine output [see Warnings and Precautions (5.2)].
Bullous and Exfoliative Skin Disorders: Inform patients of the risks of severe and exfoliative skin disorders. Instruct patients to immediately contact their healthcare provider for development of bullous lesions or exfoliation in the skin or mucous membranes [see Warnings and Precautions (5.3)].
Interstitial Pneumonitis: Inform patients of the risks of pneumonitis. Instruct patients to immediately contact their healthcare provider for development of dyspnea or persistent cough [see Warnings and Precautions (5.4)].
Radiation Recall: Inform patients who have received prior radiation of the risks of radiation recall. Instruct patients to immediately contact their healthcare provider for development of inflammation or blisters in an area that was previously irradiated [see Warnings and Precautions (5.5)].
Increased Risk of Toxicity with Ibuprofen in Patients with Renal Impairment: Advise patients with mild to moderate renal impairment of the risks associated with concomitant ibuprofen use and instruct them to avoid use of all ibuprofen containing products for 2 days before, the day of, and 2 days following administration of Pemetrexed Injection [see Dosage and Administration (2.5), Warnings and Precautions (5.6), and Drug Interactions (7)].
Embryo-Fetal Toxicity: Advise females of reproductive potential and males with female partners of reproductive potential of the potential risk to a fetus [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with Pemetrexed Injection and for 6 months after the last dose. Advise females to inform their prescriber of a known or suspected pregnancy. Advise males with female partners of reproductive potential to use effective contraception during treatment with Pemetrexed Injection and for 3 months after the last dose [see Warnings and Precautions (5.7) and Use in Specific Populations (8.3)].
Lactation: Advise women not to breastfeed during treatment with Pemetrexed Injection and for 1 week after the last dose [see Use in Specific Populations (8.2)].
