XIGDUO
These highlights do not include all the information needed to use XIGDUO XR safely and effectively. See full prescribing information for XIGDUO XR.XIGDUOXR (dapagliflozin and metformin hydrochloride extended-release) tablets, for oral useInitial U.S. Approval: 2014
ac8a0f7b-9f69-4495-abbc-3a47cd75a859
HUMAN PRESCRIPTION DRUG LABEL
Sep 12, 2023
AstraZeneca Pharmaceuticals LP
DUNS: 054743190
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
dapagliflozin and metformin hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
dapagliflozin and metformin hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
dapagliflozin and metformin hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
dapagliflozin and metformin hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
dapagliflozin and metformin hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 10 mg/1000 mg
30 Tablets NDC 0310-6280-30
xigduo® XR
(dapagliflozin/metformin HCl
extended-release) tablets
10 mg/1000 mg
Dispense with Medication Guide
Rx only
Do not crush, cut, or chew tablets.
Tablets must be swallowed whole.
AstraZeneca
Boxed Warning section
WARNING: LACTIC ACIDOSIS
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
XIGDUO XR is contraindicated in patients with:
•
Severe renal impairment (eGFR below 30 mL/min/1.73 m2), end-stage renal disease or patients on dialysis [see Warnings and Precautions (5.1)].
•
History of a serious hypersensitivity reaction to dapagliflozin, such as anaphylactic reactions or angioedema, or hypersensitivity to metformin HCl [see Adverse Reactions (6.1)].
•
Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma. Diabetic ketoacidosis should be treated with insulin [see Warnings and Precautions (5.1) and Warnings and Precautions (5.2)].
•
Severe renal impairment (eGFR below 30 mL/min/1.73 m2), end-stage renal disease or dialysis. (4)
•
History of serious hypersensitivity to dapagliflozin or hypersensitivity to metformin HCl. (4)
•
Metabolic acidosis, including diabetic ketoacidosis. (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
•
Lactic Acidosis [see Boxed Warning and Warnings and Precautions (5.1)]
•
Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis [see Warnings and Precautions (5.2)]
•
Volume Depletion [see Warnings and Precautions (5.3)]
•
Urosepsis and Pyelonephritis [see Warnings and Precautions (5.4)]
•
Use with Medications Known to Cause Hypoglycemia [see Warnings and Precautions (5.5)]
•
Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene) [see Warnings and Precautions (5.6)]
•
Vitamin B12 Concentrations [see Warnings and Precautions (5.7)]
•
Genital Mycotic Infections [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Dapagliflozin and Metformin HCl
Data from a prespecified pool of patients from 8 short-term, placebo- controlled studies of dapagliflozin coadministered with metformin immediate- or extended-release was used to evaluate safety. This pool included several add-on studies (metformin alone and in combination with a dipeptidyl peptidase-4 [DPP4] inhibitor and metformin, or insulin and metformin, 2 initial combination with metformin studies, and 2 studies of patients with CVD and type 2 diabetes mellitus who received their usual treatment [with metformin as background therapy]). For studies that included background therapy with and without metformin, only patients who received metformin were included in the 8-study placebo-controlled pool. Across these 8 studies 983 patients were treated once daily with dapagliflozin 10 mg and metformin and 1185 were treated with placebo and metformin. These 8 studies provide a mean duration of exposure of 23 weeks. The mean age of the population was 57 years and 2% were older than 75 years. Fifty-four percent (54%) of the population was male; 88% White, 6% Asian, and 3% Black or African American. At baseline, the population had diabetes for an average of 8 years, mean hemoglobin A1c (HbA1c) was 8.4%, and renal function was normal or mildly impaired in 90% of patients and moderately impaired in 10% of patients.
The overall incidence of adverse events for the 8-study, short-term, placebo- controlled pool in patients treated with dapagliflozin 10 mg and metformin was 60.3% compared to 58.2% for the placebo and metformin group. Discontinuation of therapy due to adverse events in patients who received dapagliflozin 10 mg and metformin was 4% compared to 3.3% for the placebo and metformin group. The most commonly reported events leading to discontinuation and reported in at least 3 patients treated with dapagliflozin 10 mg and metformin were renal impairment (0.7%), increased blood creatinine (0.2%), decreased renal creatinine clearance (0.2%), and urinary tract infection (0.2%).
Table 2 shows common adverse reactions associated with the use of dapagliflozin and metformin. These adverse reactions were not present at baseline, occurred more commonly on dapagliflozin and metformin than on placebo, and occurred in at least 2% of patients treated with either dapagliflozin 5 mg or dapagliflozin 10 mg.
Table 2: Adverse Reactions in Placebo-Controlled Studies Reported in ≥2% of Patients Treated with Dapagliflozin and Metformin|
Adverse Reaction |
% of Patients | ||
|---|---|---|---|
|
Pool of 8 Placebo-Controlled Studies | |||
|
Placebo and Metformin |
Dapagliflozin |
Dapagliflozin | |
| |||
|
Female genital mycotic infections* |
1.5 |
9.4 |
9.3 |
|
Nasopharyngitis |
5.9 |
6.3 |
5.2 |
|
Urinary tract infections† |
3.6 |
6.1 |
5.5 |
|
Diarrhea |
5.6 |
5.9 |
4.2 |
|
Headache |
2.8 |
5.4 |
3.3 |
|
Male genital mycotic infections‡ |
0 |
4.3 |
3.6 |
|
Influenza |
2.4 |
4.1 |
2.6 |
|
Nausea |
2.0 |
3.9 |
2.6 |
|
Back pain |
3.2 |
3.4 |
2.5 |
|
Dizziness |
2.2 |
3.2 |
1.8 |
|
Cough |
1.9 |
3.2 |
1.4 |
|
Constipation |
1.6 |
2.9 |
1.9 |
|
Dyslipidemia |
1.4 |
2.7 |
1.5 |
|
Pharyngitis |
1.1 |
2.7 |
1.5 |
|
Increased urination§ |
1.4 |
2.4 |
2.6 |
|
Discomfort with urination |
1.1 |
2.2 |
1.6 |
Metformin HCl
In placebo-controlled monotherapy trials of metformin extended-release, diarrhea and nausea/vomiting were reported in >5% of metformin-treated patients and more commonly than in placebo-treated patients (9.6% versus 2.6% for diarrhea and 6.5% versus 1.5% for nausea/vomiting). Diarrhea led to discontinuation of study medication in 0.6% of the patients treated with metformin extended-release.
Dapagliflozin
Dapagliflozin 10 mg has been evaluated in clinical trials in patients with type 2 diabetes mellitus, patients with heart failure, and patients with chronic kidney disease. The overall safety profile of dapagliflozin was consistent across the studied indications. No new adverse reactions were identified in the DAPA-HF and DAPA-CKD studies.
**Pool of 12 Placebo-Controlled Studies for Dapagliflozin 5 and 10 mg for
Glycemic Control**
Dapagliflozin
The data in Table 3 are derived from 12 glycemic control placebo-controlled studies ranging from 12 to 24 weeks. In 4 studies dapagliflozin was used as monotherapy, and in 8 studies dapagliflozin was used as add-on to background antidiabetic therapy or as combination therapy with metformin [see Clinical Studies (14.1)].
These data reflect exposure of 2338 patients to dapagliflozin with a mean exposure duration of 21 weeks. Patients received placebo (N=1393), dapagliflozin 5 mg (N=1145), or dapagliflozin 10 mg (N=1193) once daily. The mean age of the population was 55 years and 2% were older than 75 years of age. Fifty percent (50%) of the population were male; 81% were White, 14% were Asian, and 3% were Black or African American. At baseline, the population had diabetes for an average of 6 years, had a mean HbA1c of 8.3%, and 21% had established microvascular complications of diabetes. Baseline renal function was normal or mildly impaired in 92% of patients and moderately impaired in 8% of patients (mean eGFR 86 mL/min/1.73 m2).
Table 3 shows common adverse reactions associated with the use of dapagliflozin. These adverse reactions were not present at baseline, occurred more commonly on dapagliflozin than on placebo, and occurred in at least 2% of patients treated with either dapagliflozin 5 mg or dapagliflozin 10 mg.
Table 3: Adverse Reactions in Placebo-Controlled Studies of Glycemic Control Reported in ≥2% of Patients Treated with Dapagliflozin|
Adverse Reaction |
% of Patients | ||
|---|---|---|---|
|
Pool of 12 Placebo-Controlled Studies | |||
|
Placebo N=1393 |
Dapagliflozin |
Dapagliflozin | |
| |||
|
Female genital mycotic infections* |
1.5 |
8.4 |
6.9 |
|
Nasopharyngitis |
6.2 |
6.6 |
6.3 |
|
Urinary tract infections† |
3.7 |
5.7 |
4.3 |
|
Back pain |
3.2 |
3.1 |
4.2 |
|
Increased urination‡ |
1.7 |
2.9 |
3.8 |
|
Male genital mycotic infections§ |
0.3 |
2.8 |
2.7 |
|
Nausea |
2.4 |
2.8 |
2.5 |
|
Influenza |
2.3 |
2.7 |
2.3 |
|
Dyslipidemia |
1.5 |
2.1 |
2.5 |
|
Constipation |
1.5 |
2.2 |
1.9 |
|
Discomfort with urination |
0.7 |
1.6 |
2.1 |
|
Pain in extremity |
1.4 |
2.0 |
1.7 |
**Pool of 13 Placebo-Controlled Studies for Dapagliflozin 10 mg for
Glycemic Control**
Dapagliflozin 10 mg was also evaluated in a larger glycemic control placebo- controlled study pool. This pool combined 13 placebo-controlled studies, including 3 monotherapy studies, 9 add-on to background antidiabetic therapy studies, and an initial combination with metformin study. Across these 13 studies, 2360 patients were treated once daily with dapagliflozin 10 mg for a mean duration of exposure of 22 weeks. The mean age of the population was 59 years and 4% were older than 75 years. Fifty-eight percent (58%) of the population were male; 84% were White, 9% were Asian, and 3% were Black or African American. At baseline, the population had diabetes for an average of 9 years, had a mean HbA1c of 8.2%, and 30% had established microvascular disease. Baseline renal function was normal or mildly impaired in 88% of patients and moderately impaired in 11% of patients (mean eGFR 82 mL/min/1.73 m2).
Volume Depletion
Dapagliflozin causes an osmotic diuresis, which may lead to a reduction in intravascular volume. Adverse reactions related to volume depletion (including reports of dehydration, hypovolemia, orthostatic hypotension, or hypotension) for the 12-study and 13-study, short-term, placebo-controlled pools and for the DECLARE study are shown in Table 4 [see Warnings and Precautions (5.3)].
Table 4: Adverse Reactions Related to Volume Depletion* in Clinical Studies with Dapagliflozin
| |||||||
|
Pool of 12 Placebo-Controlled Studies |
Pool of 13 Placebo-Controlled Studies |
DECLARE Study | |||||
|
Placebo |
Dapagliflozin 5 mg |
Dapagliflozin 10 mg |
Placebo |
Dapagliflozin 10 mg |
Placebo |
Dapagliflozin 10 mg | |
|
Overall population N (%) |
N=1393 5 (0.4%) |
N=1145 7 (0.6%) |
N=1193 9 (0.8%) |
N=2295 17 (0.7%) |
N=2360 27 (1.1%) |
N=8569 207 (2.4%) |
N=8574 213 (2.5%) |
|
Patient Subgroup n (%) | |||||||
|
Patients on loop diuretics |
n=55 1 (1.8%) |
n=40 0 |
n=31 3 (9.7%) |
n=267 4 (1.5%) |
n=236 6 (2.5%) |
n=934 57 (6.1%) |
n=866 57 (6.6%) |
|
Patients with moderate renal impairment with eGFR ≥30 and <60 mL/min/1.73 m2 |
n=107 2 (1.9%) |
n=107 1 (0.9%) |
n=89 1 (1.1%) |
n=268 4 (1.5%) |
n=265 5 (1.9%) |
n=658 30 (4.6%) |
n=604 35 (5.8%) |
|
Patients ≥65 years of age |
n=276 1 (0.4%) |
n=216 1 (0.5%) |
n=204 3 (1.5%) |
n=711 6 (0.8%) |
n=665 11 (1.7%) |
n=3950 121 (3.1%) |
n=3948 117 (3.0%) |
Hypoglycemia
The frequency of hypoglycemia by study [see Clinical Studies (14.1)] is shown in Table 5. Hypoglycemia was more frequent when dapagliflozin was added to sulfonylurea or insulin [see Warnings and Precautions (5.5)].
Table 5: Incidence of Severe Hypoglycemia* and Hypoglycemia with Glucose <54 mg/dL† in Controlled Glycemic Control Clinical Studies
| |||
|
Placebo |
Dapagliflozin 5 mg |
Dapagliflozin 10 mg | |
|
Add-on to Metformin (24 weeks) |
N=137 |
N=137 |
N=135 |
|
Severe [n (%)] |
0 |
0 |
0 |
|
Glucose <54 mg/dL [n (%)] |
0 |
0 |
0 |
|
Add-on to DPP4 inhibitor (with or without Metformin) (24 weeks) |
N=226 |
– |
N=225 |
|
Severe [n (%)] |
0 |
– |
1 (0.4) |
|
Glucose <54 mg/dL [n (%)] |
1 (0.4) |
– |
1 (0.4) |
|
Add-on to Insulin with or without other OADs‡** (24 weeks)** |
N=197 |
N=212 |
N=196 |
|
Severe [n (%)] |
1 (0.5) |
2 (0.9) |
2 (1.0) |
|
Glucose <54 mg/dL [n (%)] |
43 (21.8) |
55 (25.9) |
45 (23.0) |
In the DECLARE study [see Clinical Studies (14.2)], severe events of hypoglycemia were reported in 58 (0.7%) out of 8574 patients treated with dapagliflozin 10 mg and 83 (1.0%) out of 8569 patients treated with placebo.
Genital Mycotic Infections
In the glycemic control studies, genital mycotic infections were more frequent with dapagliflozin treatment. Genital mycotic infections were reported in 0.9% of patients on placebo, 5.7% on dapagliflozin 5 mg, and 4.8% on dapagliflozin 10 mg, in the 12-study placebo-controlled pool. Discontinuation from study due to genital infection occurred in 0% of placebo-treated patients and 0.2% of patients treated with dapagliflozin 10 mg. Infections were more frequently reported in females than in males (see Table 3). The most frequently reported genital mycotic infections were vulvovaginal mycotic infections in females and balanitis in males. Patients with a history of genital mycotic infections were more likely to have a genital mycotic infection during the study than those with no prior history (10.0%, 23.1%, and 25.0% versus 0.8%, 5.9%, and 5.0% on placebo, dapagliflozin 5 mg, and dapagliflozin 10 mg, respectively). In the DECLARE study [see Clinical Studies (14.2)], serious genital mycotic infections were reported in <0.1% of patients treated with dapagliflozin 10 mg and <0.1% of patients treated with placebo. Genital mycotic infections that caused study drug discontinuation were reported in 0.9% of patients treated with dapagliflozin 10 mg and <0.1% of patients treated with placebo.
Hypersensitivity Reactions
Hypersensitivity reactions (e.g., angioedema, urticaria, hypersensitivity) were reported with dapagliflozin treatment. In glycemic control studies, serious anaphylactic reactions and severe cutaneous adverse reactions and angioedema were reported in 0.2% of comparator-treated patients and 0.3% of dapagliflozin-treated patients. If hypersensitivity reactions occur, discontinue use of dapagliflozin; treat per standard of care and monitor until signs and symptoms resolve.
Ketoacidosis
In the DECLARE study [see Clinical Studies (14.2)], events of diabetic ketoacidosis (DKA) were reported in 27 out of 8574 patients in the dapagliflozin-treated group and in 12 out of 8569 patients in the placebo group. The events were evenly distributed over the study period.
Laboratory Tests
Increases in Serum Creatinine and Decreases in eGFR
Dapagliflozin
Initiation of SGLT2 inhibitors, including dapagliflozin, causes a small increase in serum creatinine and decrease in eGFR. These changes in serum creatinine and eGFR generally occur within two weeks of starting therapy and then stabilize regardless of baseline kidney function. Changes that do not fit this pattern should prompt further evaluation to exclude the possibility of acute kidney injury [see Warnings and Precautions (5.3)]. In two studies that included patients with type 2 diabetes mellitus with moderate renal impairment, the acute effect on eGFR reversed after treatment discontinuation, suggesting acute hemodynamic changes may play a role in the renal function changes observed with dapagliflozin.
Increase in Hematocrit
Dapagliflozin
In the pool of 13 placebo-controlled studies of glycemic control, increases from baseline in mean hematocrit values were observed in dapagliflozin-treated patients starting at Week 1 and continuing up to Week 16, when the maximum mean difference from baseline was observed. At Week 24, the mean changes from baseline in hematocrit were -0.33% in the placebo group and 2.30% in the dapagliflozin 10 mg group. By Week 24, hematocrit values >55% were reported in 0.4% of placebo-treated patients and 1.3% of dapagliflozin 10 mg-treated patients.
Increase in Low-Density Lipoprotein Cholesterol
Dapagliflozin
In the pool of 13 placebo-controlled studies of glycemic control, changes from baseline in mean lipid values were reported in dapagliflozin-treated patients compared to placebo-treated patients. Mean percent changes from baseline at Week 24 were 0.0% versus 2.5% for total cholesterol, and -1.0% versus 2.9% for LDL cholesterol in the placebo and dapagliflozin 10 mg groups, respectively. In the DECLARE study [see Clinical Studies (14.2)], mean changes from baseline after 4 years were 0.4 mg/dL versus -4.1 mg/dL for total cholesterol, and -2.5 mg/dL versus -4.4 mg/dL for LDL cholesterol, in dapagliflozin 10 mg-treated and the placebo groups, respectively.
Vitamin B12 Concentrations
Metformin HCl
In metformin clinical trials of 29-week duration, a decrease to subnormal levels of previously normal serum vitamin B12 levels was observed in approximately 7% of patients.
6.2 Postmarketing Experience
Dapagliflozin
Additional adverse reactions have been identified during post-approval use of dapagliflozin. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections: Necrotizing fasciitis of the perineum (Fournier’s Gangrene), urosepsis and pyelonephritis
Metabolism and Nutrition Disorders: Ketoacidosis
Renal and Urinary Disorders: Acute kidney injury
Skin and Subcutaneous Tissue Disorders: Rash
Metformin HCl
Hepatobiliary Disorders: Cholestatic, hepatocellular, and mixed hepatocellular liver injury
•
Adverse reactions reported in >5% of patients treated with XIGDUO XR were female genital mycotic infection, nasopharyngitis, urinary tract infection, diarrhea, and headache. (6.1)
•
Adverse reactions reported in >5% of patients treated with metformin extended-release are: diarrhea and nausea/vomiting. (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Table 6: Clinically Relevant Interactions with XIGDUO XR|
Carbonic Anhydrase Inhibitors | |
|
Clinical Impact |
Topiramate or other carbonic anhydrase inhibitors (e.g., zonisamide, acetazolamide or dichlorphenamide) frequently causes a decrease in serum bicarbonate and induce non-anion gap, hyperchloremic metabolic acidosis. Concomitant use of these drugs with XIGDUO XR may increase the risk for lactic acidosis. |
|
Intervention |
Consider more frequent monitoring of these patients. |
|
Drugs that Reduce Metformin Clearance | |
|
Clinical Impact |
Concomitant use of drugs that interfere with common renal tubular transport systems involved in the renal elimination of metformin (e.g., organic cationic transporter-2 [OCT2]/multidrug and toxin extrusion [MATE] inhibitors, such as ranolazine, vandetanib, dolutegravir, and cimetidine) could increase systemic exposure to metformin and may increase the risk for lactic acidosis [see Clinical Pharmacology (12.3)]. |
|
Intervention |
Consider the benefits and risks of concomitant use. |
|
Alcohol | |
|
Clinical Impact |
Alcohol is known to potentiate the effect of metformin on lactate metabolism. |
|
Intervention |
Warn patients against excessive alcohol intake while receiving XIGDUO XR. |
|
Insulin or Insulin Secretagogues | |
|
Clinical Impact |
The risk of hypoglycemia may be increased when XIGDUO XR is used concomitantly with insulin or insulin secretagogues (e.g., sulfonylurea) [see Warnings and Precautions (5.5)]. |
|
Intervention |
Concomitant use may require lower doses of insulin or the insulin secretagogue to reduce the risk of hypoglycemia. |
|
Drugs Affecting Glycemic Control | |
|
Clinical Impact |
Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. These medications include thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. |
|
Intervention |
When such drugs are administered to a patient receiving XIGDUO XR, observe the patient closely for loss of blood glucose control. When such drugs are withdrawn from a patient receiving XIGDUO XR, observe the patient closely for hypoglycemia. |
|
Lithium | |
|
Clinical Impact |
Concomitant use of an SGLT2 inhibitor with lithium may decrease serum lithium concentrations. |
|
Intervention |
Monitor serum lithium concentration more frequently during XIGDUO XR initiation and dosage changes. |
|
Positive Urine Glucose Test | |
|
Clinical Impact |
SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. |
|
Intervention |
Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. |
|
Interference with 1,5-anhydroglucitol (1,5-AG) Assay | |
|
Clinical Impact |
Measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. |
|
Intervention |
Monitoring glycemic control with 1,5-AG assay is not recommended. Use alternative methods to monitor glycemic control. |
•
Carbonic anhydrase inhibitors: May increase risk of lactic acidosis. Consider more frequent monitoring. (7)
•
Drugs that reduce metformin clearance: May increase risk of lactic acidosis. Consider benefits and risks of concomitant use. (7)
•
See full prescribing information for additional drug interactions and information on interference of XIGDUO XR with laboratory tests. (7)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Indications and Usage (1) 09/2023
Dosage and Administration (2.6) 09/2023
Warnings and Precautions (5.2) 09/2023
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
XIGDUO XR (dapagliflozin and metformin HCl) extended-release tablets are available as follows:
Table 1: Dosage Forms and Strengths for XIGDUO XR|
Dapagliflozin |
Metformin HCl Strength |
Color/Shape |
Tablet Markings |
|
2.5 mg |
1,000 mg |
light brown to brown, biconvex, oval-shaped, and film-coated tablet |
"1074" and "2.5/1000" debossed on one side and plain on the reverse side |
|
5 mg |
500 mg |
orange, biconvex, capsule-shaped, and film-coated tablet |
"1070" and "5/500" debossed on one side and plain on the reverse side |
|
5 mg |
1,000 mg |
pink to dark pink, biconvex, oval-shaped, and film-coated tablet |
"1071" and "5/1000" debossed on one side and plain on the reverse side |
|
10 mg |
500 mg |
pink, biconvex, capsule-shaped, and film-coated tablet |
"1072" and "10/500" debossed on one side and plain on the reverse side |
|
10 mg |
1,000 mg |
yellow to dark yellow, biconvex, oval-shaped, and film-coated tablet |
"1073" and "10/1000" debossed on one side and plain on the reverse side |
•
2.5 mg dapagliflozin/1,000 mg metformin HCl extended-release (3)
•
5 mg dapagliflozin/500 mg metformin HCl extended-release (3)
•
5 mg dapagliflozin/1,000 mg metformin HCl extended-release (3)
•
10 mg dapagliflozin/500 mg metformin HCl extended-release (3)
•
10 mg dapagliflozin/1,000 mg metformin HCl extended-release (3)
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
There have been no clinical efficacy studies conducted with XIGDUO XR combination tablets to characterize its effect on HbA1c reduction. XIGDUO XR is considered to be bioequivalent to coadministered dapagliflozin and metformin HCl extended-release (XR) tablets [see Clinical Pharmacology (12.3)]. Relative bioavailability studies between XIGDUO XR and coadministered dapagliflozin and metformin HCl immediate-release (IR) tablets have not been conducted. The metformin HCl XR tablets and metformin HCl IR tablets have a similar extent of absorption (as measured by AUC), while peak plasma levels of XR tablets are approximately 20% lower than those of IR tablets at the same dose.
14.1 Glycemic Control
The coadministration of dapagliflozin and metformin XR tablets has been studied in treatment-naive patients inadequately controlled on diet and exercise alone. The coadministration of dapagliflozin and metformin IR or XR tablets has been studied in patients with type 2 diabetes mellitus inadequately controlled on metformin and compared with a sulfonylurea (glipizide) in combination with metformin. Treatment with dapagliflozin plus metformin at all doses produced clinically relevant and statistically significant improvements in HbA1c and fasting plasma glucose (FPG) compared to placebo in combination with metformin (initial or add-on therapy). HbA1c reductions were seen across subgroups including gender, age, race, duration of disease, and baseline body mass index (BMI).
Initial Combination Therapy with Metformin Extended-Release
A total of 1236 treatment-naive patients with inadequately controlled type 2 diabetes mellitus (HbA1c ≥7.5% and ≤12%) participated in 2 active-controlled studies of 24-week duration to evaluate initial therapy with dapagliflozin 5 mg (NCT00643851) or 10 mg (NCT00859898) in combination with metformin extended-release (XR) formulation.
In one study, 638 patients randomized to 1 of 3 treatment arms following a 1-week lead-in period received: dapagliflozin 10 mg plus metformin XR (up to 2000 mg/day), dapagliflozin 10 mg plus placebo, or metformin XR (up to 2000 mg/day) plus placebo. Metformin XR dose was up-titrated weekly in 500 mg increments, as tolerated, with a median dose achieved of 2000 mg.
The combination treatment of dapagliflozin 10 mg plus metformin XR provided statistically significant improvements in HbA1c and FPG compared with either of the monotherapy treatments and statistically significant reduction in body weight compared with metformin XR alone (see Table 11 and Figure 2). Dapagliflozin 10 mg as monotherapy also provided statistically significant improvements in FPG and statistically significant reduction in body weight compared with metformin alone and was non-inferior to metformin XR monotherapy in lowering HbA1c.
Table 11: Results at Week 24 (LOCF*) in an Active-Controlled Study of Dapagliflozin Initial Combination Therapy with Metformin XR
| |||
|
Efficacy Parameter |
Dapagliflozin 10 mg + Metformin XR N=211† |
Dapagliflozin 10 mg N=219† |
Metformin XR N=208† |
|
HbA1c (%) | |||
|
Baseline (mean) |
9.1 |
9.0 |
9.0 |
|
Change from baseline (adjusted mean‡) |
-2.0 |
-1.5 |
-1.4 |
|
Difference from dapagliflozin (adjusted mean‡) (95% CI) |
-0.5§ (-0.7, -0.3) | ||
|
Difference from metformin XR (adjusted mean‡) (95% CI) |
-0.5§ (-0.8, -0.3) |
0.0¶ (-0.2, 0.2) | |
|
Percent of patients achieving HbA1c <7% adjusted for baseline |
46.6% |
31.7% |
35.2% |
|
FPG (mg/dL) | |||
|
Baseline (mean) |
189.6 |
197.5 |
189.9 |
|
Change from baseline (adjusted mean‡) |
-60.4 |
-46.4 |
-34.8 |
|
Difference from dapagliflozin (adjusted mean‡) (95% CI) |
-13.9§ (-20.9, -7.0) | ||
|
Difference from metformin XR (adjusted mean‡) (95% CI) |
-25.5§ (-32.6, -18.5) |
-11.6# (-18.6, -4.6) | |
|
Body Weight (kg) | |||
|
Baseline (mean) |
88.6 |
88.5 |
87.2 |
|
Change from baseline (adjusted mean‡) |
-3.3 |
-2.7 |
-1.4 |
|
Difference from metformin XR (adjusted mean‡) (95% CI) |
-2.0§ (-2.6, -1.3) |
-1.4§ (-2.0, -0.7) |
Figure 2: Adjusted Mean Change from Baseline Over Time in HbA1c (%) in a 24-Week Active-Controlled Study of Dapagliflozin Initial Combination Therapy with Metformin XR
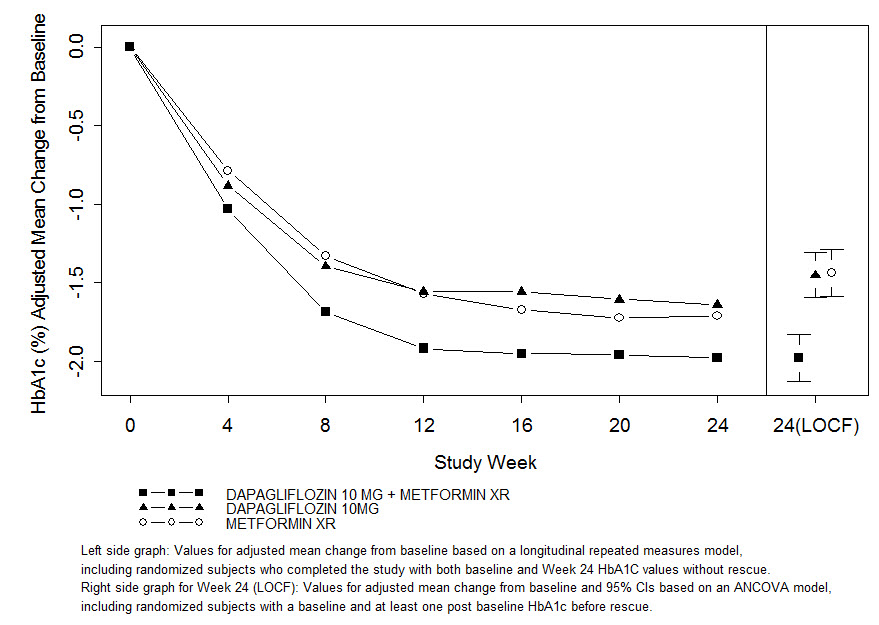
In the second study, 603 patients were randomized to 1 of 3 treatment arms following a 1-week lead-in period: dapagliflozin 5 mg plus metformin XR (up to 2000 mg/day), dapagliflozin 5 mg plus placebo, or metformin XR (up to 2000 mg/day) plus placebo. Metformin XR dose was up-titrated weekly in 500 mg increments, as tolerated, with a median dose achieved of 2000 mg.
The combination treatment of dapagliflozin 5 mg plus metformin XR provided statistically significant improvements in HbA1c and FPG compared with either of the monotherapy treatments and statistically significant reduction in body weight compared with metformin XR alone (see Table 12).
Table 12: Results at Week 24 (LOCF*) in an Active-Controlled Study of Dapagliflozin Initial Combination Therapy with Metformin XR
| |||
|
Efficacy Parameter |
Dapagliflozin 5 mg + Metformin XR N=194† |
Dapagliflozin 5 mg N=203† |
Metformin XR N=201† |
|
HbA1c (%) | |||
|
Baseline (mean) |
9.2 |
9.1 |
9.1 |
|
Change from baseline (adjusted mean‡) |
-2.1 |
-1.2 |
-1.4 |
|
Difference from dapagliflozin (adjusted mean‡) (95% CI) |
-0.9§ (-1.1, -0.6) | ||
|
Difference from metformin XR (adjusted mean‡) (95% CI) |
-0.7§ (-0.9, -0.5) | ||
|
Percent of patients achieving HbA1c <7% adjusted for baseline |
52.4%¶ |
22.5% |
34.6% |
|
FPG (mg/dL) | |||
|
Baseline (mean) |
193.4 |
190.8 |
196.7 |
|
Change from baseline (adjusted mean‡) |
-61.0 |
-42.0 |
-33.6 |
|
Difference from dapagliflozin (adjusted mean‡) (95% CI) |
-19.1§ (-26.7, -11.4) | ||
|
Difference from metformin XR (adjusted mean‡) (95% CI) |
-27.5§ (-35.1, -19.8) | ||
|
Body Weight (kg) | |||
|
Baseline (mean) |
84.2 |
86.2 |
85.8 |
|
Change from baseline (adjusted mean‡) |
-2.7 |
-2.6 |
-1.3 |
|
Difference from metformin XR (adjusted mean‡) (95% CI) |
-1.4§ (-2.0, -0.7) |
Add-On to Metformin Immediate-Release
A total of 546 patients with type 2 diabetes mellitus with inadequate glycemic control (HbA1c ≥7% and ≤10%) participated in a 24-week, placebo-controlled study to evaluate dapagliflozin in combination with metformin (NCT00528879). Patients on metformin at a dose of at least 1500 mg/day were randomized after completing a 2-week, single-blind, placebo lead-in period. Following the lead- in period, eligible patients were randomized to dapagliflozin 5 mg, dapagliflozin 10 mg, or placebo in addition to their current dose of metformin.
As add-on treatment to metformin, dapagliflozin 10 mg provided statistically significant improvements in HbA1c and FPG, and statistically significant reduction in body weight compared with placebo at Week 24 (see Table 13 and Figure 3). Statistically significant (p<0.05 for both doses) mean changes from baseline in systolic blood pressure relative to placebo plus metformin were -4.5 mmHg and -5.3 mmHg with dapagliflozin 5 mg and 10 mg plus metformin, respectively.
Table 13: Results of a 24-Week (LOCF*) Placebo-Controlled Study of Dapagliflozin in Add-On Combination with Metformin
| |||
|
Efficacy Parameter |
Dapagliflozin 10 mg + Metformin N=135† |
Dapagliflozin 5 mg + Metformin N=137† |
Placebo + Metformin N=137† |
|
HbA1c (%) | |||
|
Baseline (mean) |
7.9 |
8.2 |
8.1 |
|
Change from baseline (adjusted mean‡) |
-0.8 |
-0.7 |
-0.3 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-0.5§ (-0.7, -0.3) |
-0.4§ (-0.6, -0.2) | |
|
Percent of patients achieving HbA1c <7% adjusted for baseline |
40.6%¶ |
37.5%¶ |
25.9% |
|
FPG (mg/dL) | |||
|
Baseline (mean) |
156.0 |
169.2 |
165.6 |
|
Change from baseline at Week 24 (adjusted mean‡) |
-23.5 |
-21.5 |
-6.0 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-17.5§ (-25.0, -10.0) |
-15.5§ (-22.9, -8.1) | |
|
Change from baseline at Week 1 (adjusted mean‡) |
-16.5§ (N=115) |
-12.0§ (N=121) |
1.2 |
|
Body Weight (kg) | |||
|
Baseline (mean) |
86.3 |
84.7 |
87.7 |
|
Change from baseline (adjusted mean‡) |
-2.9 |
-3.0 |
-0.9 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-2.0§ (-2.6, -1.3) |
-2.2§ (-2.8, -1.5) |
Figure 3: Adjusted Mean Change from Baseline Over Time in HbA1c (%) in a 24-Week Placebo-Controlled Study of Dapagliflozin in Combination with Metformin
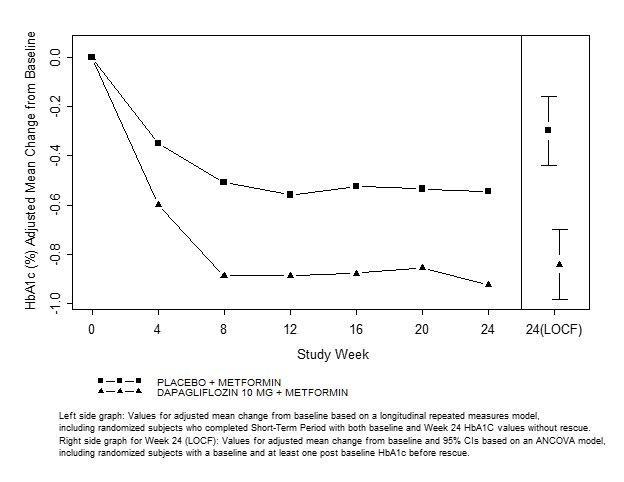
**Active Glipizide-Controlled Study Add-On to Metformin Immediate-
Release**
A total of 816 patients with type 2 diabetes mellitus with inadequate glycemic control (HbA1c >6.5% and ≤10%) were randomized in a 52-week, glipizide- controlled, non-inferiority study to evaluate dapagliflozin as add-on therapy to metformin (NCT00660907). Patients on metformin at a dose of at least 1500 mg/day were randomized following a 2-week placebo lead-in period to glipizide or dapagliflozin (5 mg or 2.5 mg, respectively) and were up-titrated over 18 weeks to optimal glycemic effect (FPG <110 mg/dL, <6.1 mmol/L) or to the highest dose level (up to glipizide 20 mg and dapagliflozin 10 mg) as tolerated by patients. Thereafter, doses were kept constant, except for down- titration to prevent hypoglycemia.
At the end of the titration period, 87% of patients treated with dapagliflozin had been titrated to the maximum study dose (10 mg) versus 73% treated with glipizide (20 mg). Dapagliflozin treatment led to a similar mean reduction in HbA1c from baseline at Week 52 (LOCF), compared with glipizide, thus demonstrating non-inferiority (see Table 14). Dapagliflozin treatment led to a statistically significant mean reduction in body weight from baseline at Week 52 (LOCF) compared with a mean increase in body weight in the glipizide group. Statistically significant (p<0.0001) mean change from baseline in systolic blood pressure relative to glipizide plus metformin was −5.0 mmHg with dapagliflozin plus metformin.
Table 14: Results at Week 52 (LOCF*) in an Active-Controlled Study Comparing Dapagliflozin to Glipizide as Add-On to Metformin
| ||
|
Efficacy Parameter |
Dapagliflozin + Metformin N=400† |
Glipizide + Metformin N=401† |
|
HbA1c (%) | ||
|
Baseline (mean) |
7.7 |
7.7 |
|
Change from baseline (adjusted mean‡) |
-0.5 |
-0.5 |
|
Difference from glipizide + metformin (adjusted mean‡) (95% CI) |
0.0§ (-0.1, 0.1) | |
|
Body Weight (kg) | ||
|
Baseline (mean) |
88.4 |
87.6 |
|
Change from baseline (adjusted mean‡) |
-3.2 |
1.4 |
|
Difference from glipizide + metformin (adjusted mean‡) (95% CI) |
-4.7¶ (-5.1, -4.2) |
**Use in Patients with Type 2 Diabetes Mellitus and Moderate Renal
Impairment**
Dapagliflozin was assessed in two placebo-controlled studies of patients with type 2 diabetes mellitus and moderate renal impairment.
Patients with type 2 diabetes mellitus and an eGFR between 45 to less than 60 mL/min/1.73 m2 inadequately controlled on current diabetes therapy participated in a 24-week, double-blind, placebo-controlled clinical study (NCT02413398). Patients were randomized to either dapagliflozin 10 mg or placebo, administered orally once daily. At Week 24, dapagliflozin provided statistically significant reductions in HbA1c compared with placebo (Table 15).
Table 15: Results at Week 24 of Placebo-Controlled Study for Dapagliflozin in Patients with Type 2 Diabetes Mellitus and Renal Impairment (eGFR 45 to less than 60 mL/min/1.73 m2)
| ||
|
Dapagliflozin 10 mg |
Placebo | |
|
Number of patients: |
N=160 |
N=161 |
|
HbA1c (%) | ||
|
Baseline (mean) |
8.3 |
8.0 |
|
Change from baseline (adjusted mean*) |
-0.4 |
-0.1 |
|
Difference from placebo (adjusted mean*) (95% CI) |
-0.3† (-0.5, -0.1) |
14.2 Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus
Dapagliflozin Effect on Cardiovascular Events (DECLARE, NCT01730534) was an international, multicenter, randomized, double-blind, placebo-controlled, clinical study conducted to determine the effect of dapagliflozin 10 mg relative to placebo on cardiovascular (CV) outcomes when added to current background therapy. All patients had type 2 diabetes mellitus and either established CV disease or two or more additional CV risk factors (age ≥55 years in men or ≥60 years in women and one or more of dyslipidemia, hypertension, or current tobacco use). Concomitant antidiabetic and atherosclerotic therapies could be adjusted, at the discretion of investigators, to ensure participants were treated according to the standard care for these diseases.
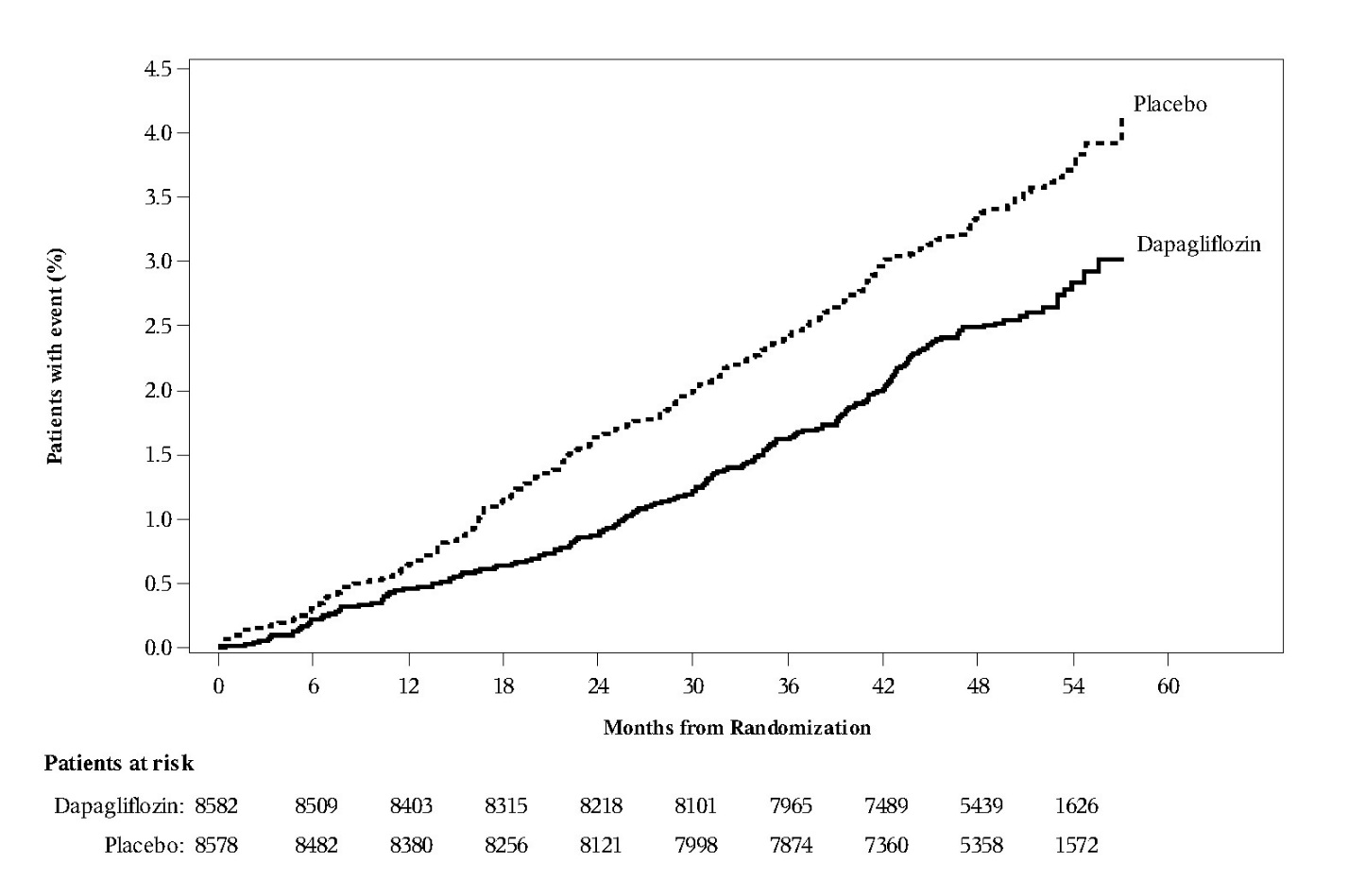
Of 17160 randomized patients, 6974 (40.6%) had established CV disease and 10186 (59.4%) did not have established CV disease. A total of 8582 patients were randomized to dapagliflozin 10 mg, 8578 to placebo, and patients were followed for a median of 4.2 years.
Approximately 80% of the trial population was White, 4% Black or African American, and 13% Asian. The mean age was 64 years, and approximately 63% were male.
Mean duration of diabetes was 11.9 years and 22.4% of patients had diabetes for less than 5 years. Mean eGFR was 85.2 mL/min/1.73 m2. At baseline, 23.5% of patients had microalbuminuria (UACR ≥30 to ≤300 mg/g) and 6.8% had macroalbuminuria (UACR >300 mg/g). Mean HbA1c was 8.3% and mean BMI was 32.1 kg/m2. At baseline, 10% of patients had a history of heart failure.
Most patients (98.1%) used one or more antihyperglycemic medications at baseline. 82.0% of the patients were being treated with metformin, 40.9% with insulin, 42.7% with a sulfonylurea, 16.8% with a DPP4 inhibitor, and 4.4% with a GLP-1 receptor agonist.
Approximately 81.3% of patients were treated with angiotensin converting enzyme inhibitors or angiotensin receptor blockers, 75.0% with statins, 61.1% with antiplatelet therapy, 55.5% with acetylsalicylic acid, 52.6% with beta- blockers, 34.9% with calcium channel blockers, 22.0% with thiazide diuretics, and 10.5% with loop diuretics.
A Cox proportional hazards model was used to test for non-inferiority against the pre-specified risk margin of 1.3 for the hazard ratio (HR) of the composite of CV death, myocardial infarction (MI), or ischemic stroke (MACE) and if non-inferiority was demonstrated, to test for superiority on the two primary endpoints: 1) the composite of hospitalization for heart failure or CV death, and 2) MACE.
The incidence rate of MACE was similar in both treatment arms: 2.30 MACE events per 100 patient-years on dapagliflozin vs 2.46 MACE events per 100 patient-years on placebo. The estimated hazard ratio of MACE associated with dapagliflozin relative to placebo was 0.93 with a 95% CI of (0.84, 1.03). The upper bound of this confidence interval, 1.03, excluded the pre-specified non- inferiority margin of 1.3.
Dapagliflozin 10 mg was superior to placebo in reducing the incidence of the primary composite endpoint of hospitalization for heart failure or CV death (HR 0.83 [95% CI 0.73, 0.95]).
The treatment effect was due to a significant reduction in the risk of hospitalization for heart failure in subjects randomized to dapagliflozin 10 mg (HR 0.73 [95% CI 0.61, 0.88]), with no change in the risk of CV death (Table 16 and Figures 4 and 5).
Table 16: Treatment Effects for the Primary Endpoints* and their Components* in the DECLARE Study
| |||
|
Patients with events n(%) | |||
|
Efficacy Variable (time to first occurrence) |
Dapagliflozin 10 mg N=8582 |
Placebo N=8578 |
Hazard Ratio (95% CI) |
|
Primary Endpoints | |||
|
Composite of Hospitalization for Heart Failure, CV Death† |
417 (4.9) |
496 (5.8) |
0.83 (0.73, 0.95) |
|
Composite Endpoint of CV Death, MI, Ischemic Stroke |
756 (8.8) |
803 (9.4) |
0.93 (0.84, 1.03) |
|
Components of the composite endpoints‡ | |||
|
Hospitalization for Heart Failure |
212 (2.5) |
286 (3.3) |
0.73 (0.61, 0.88) |
|
CV Death |
245 (2.9) |
249 (2.9) |
0.98 (0.82, 1.17) |
|
Myocardial Infarction |
393 (4.6) |
441 (5.1) |
0.89 (0.77, 1.01) |
|
Ischemic Stroke |
235 (2.7) |
231 (2.7) |
1.01 (0.84, 1.21) |
|
N=Number of patients, CI=Confidence interval, CV=Cardiovascular, MI=Myocardial infarction, eGFR=estimated glomerular filtration rate, ESRD=End-stage renal disease |
Figure 4: Time to First Occurrence of Hospitalization for Heart Failure or CV Death in the DECLARE Study
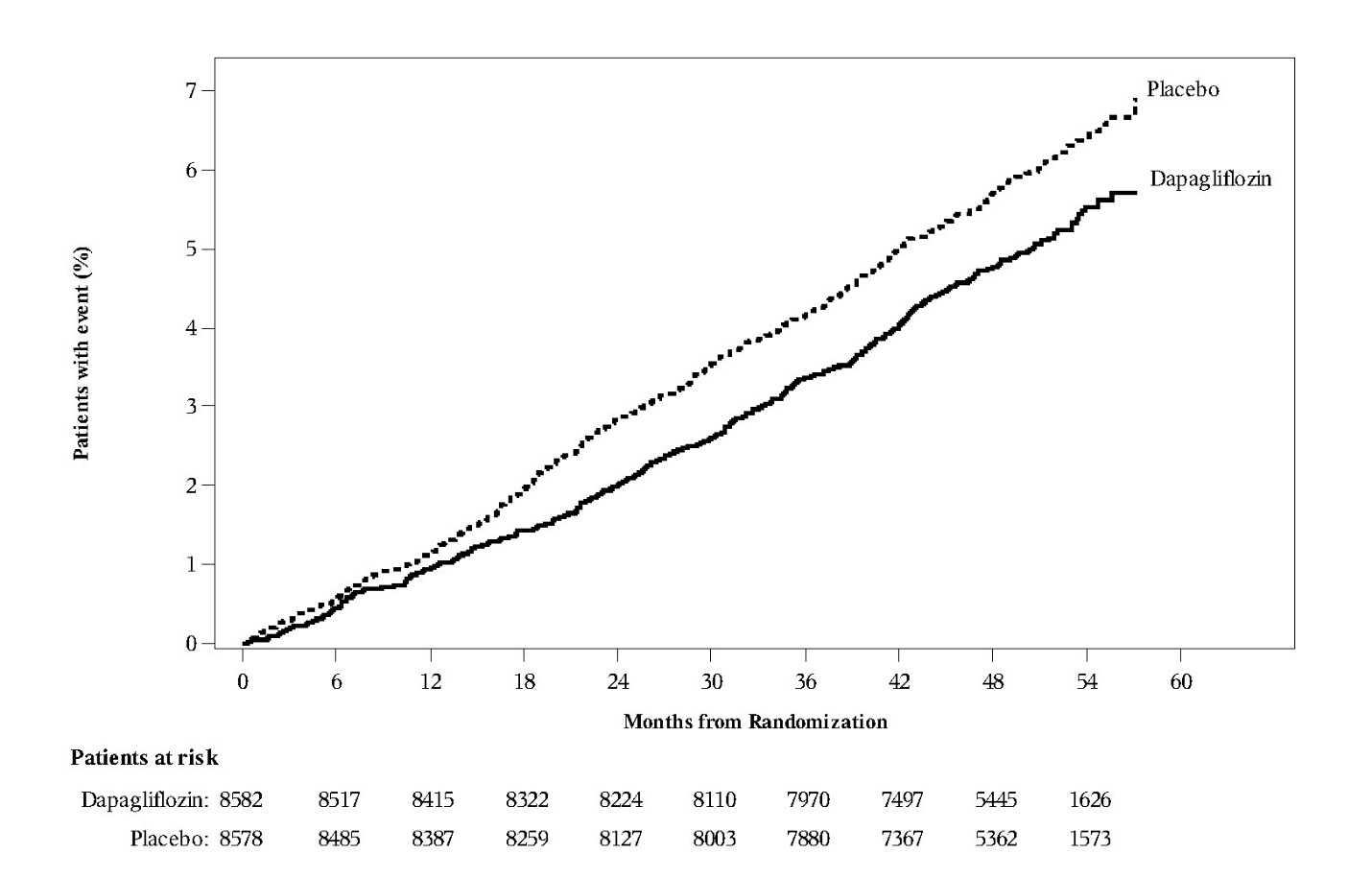
Figure 5: Time to First Occurrence of Hospitalization for Heart Failure in the DECLARE Study
14.3 Heart Failure with Reduced Ejection Fraction
Dapagliflozin And Prevention of Adverse outcomes in Heart Failure (DAPA-HF, NCT03036124) was an international, multicenter, randomized, double-blind, placebo-controlled study in patients with heart failure (New York Heart Association [NYHA] functional class II-IV) with reduced ejection fraction (left ventricular ejection fraction [LVEF] 40% or less) to determine whether dapagliflozin reduces the risk of cardiovascular death and hospitalization for heart failure.
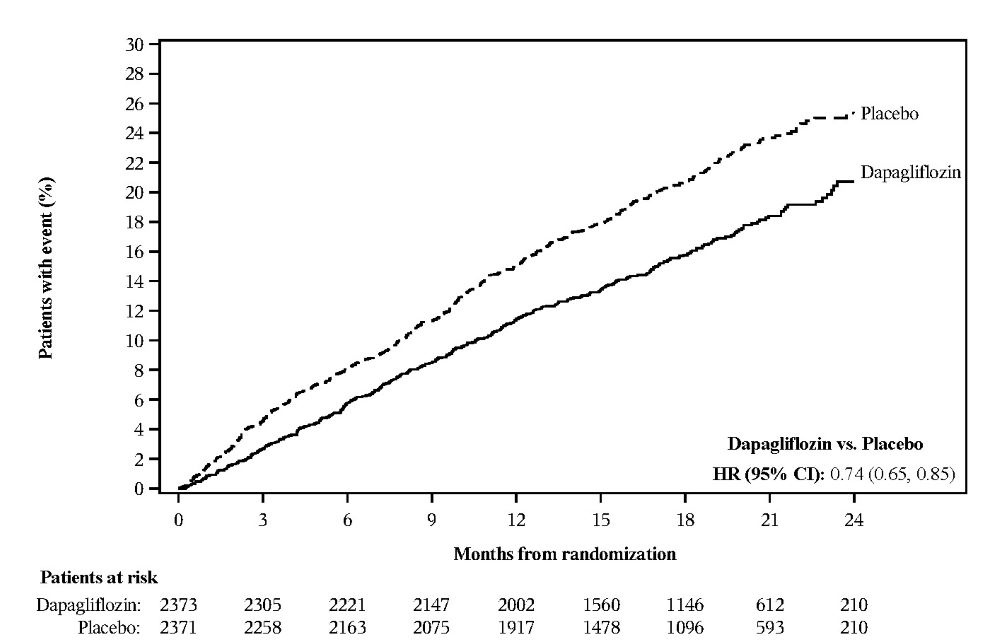
Of 4744 patients, 2373 were randomized to dapagliflozin 10 mg and 2371 to placebo and were followed for a median of 18 months. The study included patients with type 2 diabetes mellitus (n=2139) and patients without diabetes (n=2605). The mean age of the study population was 66 years, 77% were male and 70% were White, 5% Black or African American, and 24% Asian. At baseline, 68% patients were classified as NYHA class II, 32% class III, and 1% class IV; median LVEF was 32%. At baseline, 94% of patients were treated with ACEi, ARB or angiotensin receptor-neprilysin inhibitor (ARNI, including sacubitril/valsartan 11%), 96% with beta-blocker, 71% with mineralocorticoid receptor antagonist (MRA), 93% with diuretic, and 26% had an implantable device (with defibrillator function). Patients with eGFR 30 mL/min/1.73 m2 or greater at enrollment were included in the study.
History of type 2 diabetes mellitus was present in 42%, and an additional 3% had type 2 diabetes mellitus based on a HbA1c ≥6.5% at both enrollment and randomization, totaling to 1075 patients in the dapagliflozin group and 1064 in the placebo group. At baseline of the patients with type 2 diabetes mellitus, 48% were treated with metformin (505 patients on dapagliflozin 10 mg and 515 on placebo) and 25% were treated with insulin.
The mean age of the type 2 diabetes mellitus population was 67 years, 78% were male, 70% White, 6% Black or African American and 23% Asian. At baseline, 64% patients were classified as NYHA class II, 35% class III and 1% class IV, median LVEF was 32%. Patients were on standard of care therapy; 93% of type 2 diabetes mellitus patients were treated with ACEi, ARB, or angiotensin receptor-neprilysin inhibitor (ARNI, 11%), 97% with beta-blocker, 71% with mineralocorticoid receptor antagonist (MRA), 95% with diuretic and 27% had an implantable device (with defibrillator function). In these patients, mean eGFR was 63 mL/min/1.73 m2.
Dapagliflozin 10 mg reduced the incidence of the primary composite endpoint of CV death, hospitalization for heart failure or urgent heart failure visit in overall population (HR 0.74 [95% CI 0.65, 0.85]; p<0.0001). All three components of the primary composite endpoint individually contributed to the treatment effect. There were few urgent heart failure visits. The Kaplan–Meier curves for dapagliflozin 10 mg and placebo separated early and continued to diverge over the study period (Table 17 and Figure 6).
The treatment benefit of dapagliflozin 10 mg in reducing the incidence of the primary composite endpoint was consistent in patients with type 2 diabetes mellitus (HR 0.75 [95% CI 0.63, 0.90]), and in patients with type 2 diabetes mellitus and metformin as background therapy (HR 0.67 [95% CI 0.51, 0.88]).
Table 17: Treatment Effects for the Primary Composite Endpoint*, its Components*, and Secondary Endpoints in the DAPA-HF Study
| ||||
|
Patients with events (event rate†) | ||||
|
Efficacy Variable (time to first occurrence) |
Dapagliflozin 10 mg |
Placebo |
Hazard ratio |
p-value‡ |
|
Composite of hHF, CV Death or Urgent Heart Failure Visit§ |
386 (11.6) |
502 (15.6) |
0.74 (0.65, 0.85) |
<0.0001 |
|
Composite of CV Death or hHF |
382 (11.4) |
495 (15.3) |
0.75 |
<0.0001 |
|
Components of the Composite Endpoints† | ||||
|
227 (6.5) |
273 (7.9) |
0.82 | |
|
237 (7.1) |
326 (10.1) |
0.70 | |
|
231 (6.9) |
318 (9.8) |
0.70 | |
|
10 (0.3) |
23 (0.7) |
0.43 | |
|
All-Cause Mortality |
276 (7.9) |
329 (9.5) |
0.83 | |
|
N=Number of patients, CI=Confidence interval, CV=Cardiovascular, hHF=hospitalization for heart failure NOTE: Hazard Ratio based on Cox proportional hazards model with treatment as a factor, stratified by T2DM status at randomization, and adjusted for history of hHF (except for the analysis of all-cause mortality). The number of first events for the single components are the actual number of first events for each component and does not add up to the number of events in the composite endpoint. |
Figure 6: Time to the First Occurrence of the Composite of Cardiovascular Death, Hospitalization for Heart Failure or Urgent Heart Failure Visit in the DAPA-HF Study
14.4 Chronic Kidney Disease
The Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with Chronic Kidney Disease (DAPA-CKD, NCT03036150) was an international, multicenter, randomized, double-blind, placebo-controlled study in patients with chronic kidney disease (CKD) (eGFR between 25 and 75 mL/min/1.73 m2) and albuminuria (urine albumin creatinine ratio [UACR] between 200 and 5000 mg/g) who were receiving standard of care background therapy, including a maximally tolerated, labeled daily dose of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB). The trial excluded patients with autosomal dominant or autosomal recessive polycystic kidney disease, lupus nephritis, or ANCA-associated vasculitis and patients requiring cytotoxic, immunosuppressive, or immunomodulatory therapies in the preceding 6 months.
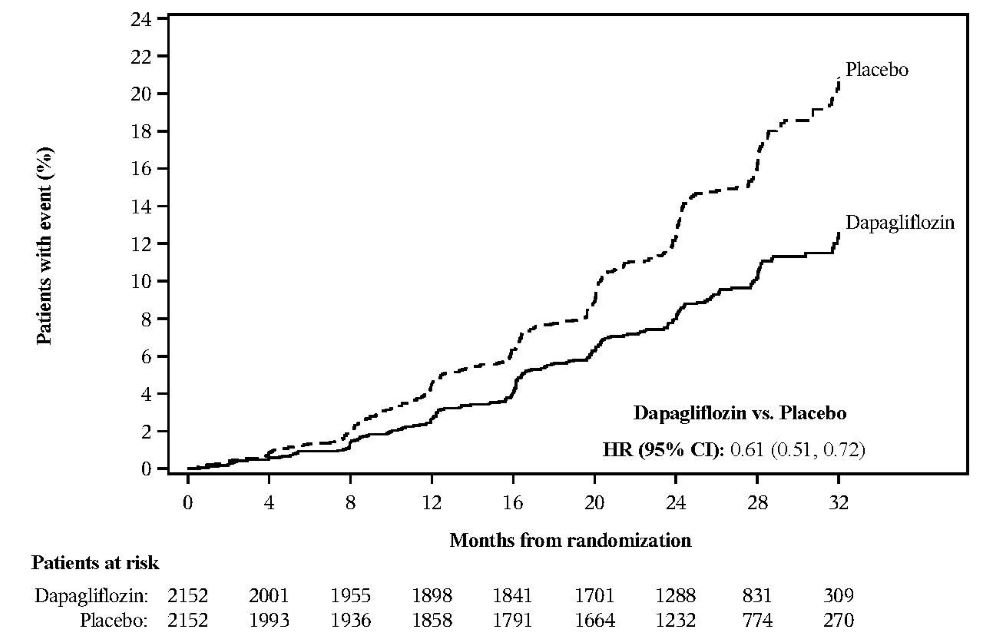
The primary objective was to determine whether dapagliflozin 10 mg reduces the incidence of the composite endpoint of ≥50% sustained decline in eGFR, progression to end-stage kidney disease (ESKD) (defined as sustained eGFR<15 mL/min/1.73 m2, initiation of chronic dialysis treatment or renal transplant), CV or renal death.
A total of 4304 patients were randomized equally to dapagliflozin 10 mg or placebo and were followed for a median of 28.5 months. The study included patients with type 2 diabetes mellitus (n=2906) and patients without diabetes (n=1398). The mean age of the study population was 62 years and 67% were male. The population was 53% White, 4% Black or African American, and 34% Asian; 25% were of Hispanic or Latino ethnicity. At baseline, mean eGFR was 43 mL/min/1.73 m2, 44% of patients had an eGFR 30 mL/min/1.73m2 to less than 45 mL/min/1.73m2, and 15% of patients had an eGFR less than 30 mL/min/1.73m2. Median UACR was 950 mg/g. The most common etiologies of CKD were diabetic nephropathy (58%), ischemic/hypertensive nephropathy (16%), and IgA nephropathy (6%). At baseline, 97% of patients were treated with ACEi or ARB. Approximately 44% were taking antiplatelet agents, and 65% were on a statin.
Out of 2906 (68%) patients who had type 2 diabetes mellitus at randomization, 1455 patients received dapagliflozin 10 mg and 1451 received placebo. At baseline of the patients with type 2 diabetes mellitus, 43% were being treated with metformin (631 patients on dapagliflozin 10 mg and 613 on placebo) and 55% were treated with insulin.
The mean age of the type 2 diabetes mellitus study population was 64 years, 67% were male, 53% White, 5% Black or African American and 32% Asian, 27% were of Hispanic or Latino ethnicity. In these patients, mean eGFR was 44 mL/min/1.73 m2, 43% of patients had an eGFR 30 mL/min/1.73 m2 to below 45 mL/min/1.73 m2, and 14% of patients had an eGFR below 30 mL/min/1.73 m2. Median UACR was 1017 mg/g. The most common etiologies of CKD in this group were diabetic nephropathy (86%) and ischemic/hypertensive nephropathy (7%).
Dapagliflozin 10 mg reduced the incidence of the primary composite endpoint of ≥50% sustained decline in eGFR, progression to ESKD, CV or renal death in overall population (HR 0.61 [95% CI 0.51,0.72]; p<0.0001). The dapagliflozin 10 mg and placebo event curves separate by Month 4 and continue to diverge over the study period. The treatment effect reflected a reduction in ≥50% sustained decline in eGFR, progression to ESKD, and CV death. There were few renal deaths during the trial (Table 18 and Figure 7).
The treatment benefit of dapagliflozin 10 mg was consistent in reducing the incidence of the primary composite endpoint in patients with type 2 diabetes mellitus (HR 0.64 [95% CI 0.52, 0.79]) and in patients with type 2 diabetes mellitus and metformin as background therapy (HR 0.74 [95% CI 0.53, 1.03]).
The treatment benefit of dapagliflozin 10 mg was consistent in reducing the incidence of the composite endpoint of CV death or hospitalization for heart failure and all-cause mortality in patients with type 2 diabetes mellitus (HR 0.70 [95% CI 0.53, 0.92] and HR 0.74 [95% CI 0.56, 0.98], respectively) and in patients with type 2 diabetes mellitus and metformin as background therapy (HR 0.59 [95% CI 0.38, 0.91] and HR 0.71 [95% CI 0.46, 1.10]).
Table 18: Treatment Effect for the Primary Composite Endpoint, its Components, and Secondary Composite Endpoints in DAPA-CKD Study
| ||||
|
Patients with events (event rate) | ||||
|
Efficacy Variable (time to first occurrence) |
Dapagliflozin 10 mg |
Placebo |
Hazard ratio |
p-value |
|
Composite of ≥50% sustained eGFR decline, ESKD, CV or renal death |
197 (4.6) |
312 (7.5) |
0.61 |
<0.0001 |
|
Components of the primary composite endpoint | ||||
|
≥50% Sustained eGFR Decline |
112 (2.6) |
201 (4.8) |
0.53 | |
|
ESKD* |
109 (2.5) |
161 (3.8) |
0.64 | |
|
CV Death |
65 (1.4) |
80 (1.7) |
0.81 | |
|
Renal Death |
2 (0.0) |
6 (0.1) | ||
|
≥50% sustained eGFR decline, ESKD or renal death |
142 (3.3) |
243 (5.8) |
0.56 |
<0.0001 |
|
CV death or Hospitalization for Heart Failure |
100 (2.2) |
138 (3.0) |
0.71 |
0.0089 |
|
Hospitalization for Heart Failure |
37 (0.8) |
71 (1.6) |
0.51 | |
|
All-Cause Mortality |
101 (2.2) |
146 (3.1) |
0.69 |
0.0035 |
|
N=Number of patients, CI=Confidence interval, CV=Cardiovascular. NOTE: Time to first event was analyzed in a Cox proportional hazards model. Event rates are presented as the number of subjects with event per 100 patient years of follow-up. There were too few events of renal death to compute a reliable hazard ratio. |
Figure 7: Time to First Occurrence of the Primary Composite Endpoint, ≥50% Sustained Decline in eGFR, ESKD, CV or Renal Death (DAPA-CKD Study)
DAPA-CKD enrolled a population with relatively advanced CKD at high risk of progression. Exploratory analyses of a randomized, double-blind, placebo- controlled trial conducted to determine the effect of dapagliflozin 10 mg on CV outcomes (the DECLARE trial) support the conclusion that dapagliflozin 10 mg is also likely to be effective in patients with less advanced CKD.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Lactic Acidosis
Inform patients of the risks of lactic acidosis due to the metformin component and its symptoms and conditions that predispose to its development [see Warnings and Precautions (5.1)]. Advise patients to discontinue XIGDUO XR immediately and to promptly notify their healthcare provider if unexplained hyperventilation, myalgia, malaise, unusual somnolence, dizziness, slow or irregular heartbeat, sensation of feeling cold (especially in the extremities), or other non-specific symptoms occur. Gastrointestinal symptoms are common during initiation of metformin treatment and may occur during initiation of XIGDUO XR therapy; however, inform patients to consult their physician if they develop unexplained symptoms. Although gastrointestinal symptoms that occur after stabilization are unlikely to be drug related, such an occurrence of symptoms should be evaluated to determine if it may be due to lactic acidosis or other serious disease.
Counsel patients against excessive alcohol intake while receiving XIGDUO XR [see Warnings and Precautions (5.1)].
Inform patients about the importance of regular testing of renal function and hematological parameters when receiving treatment with XIGDUO XR [see Contraindications (4) and Warnings and Precautions (5.1)].
Instruct patients to inform their healthcare provider that they are taking XIGDUO XR prior to any surgical or radiological procedure, as temporary discontinuation of XIGDUO XR may be required until renal function has been confirmed to be normal [see Warnings and Precautions (5.1)].
Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
Inform patients that XIGDUO XR can cause potentially fatal ketoacidosis and that type 2 diabetes mellitus and pancreatic disorders (e.g., history of pancreatitis or pancreatic surgery) are risk factors.
Educate all patients on precipitating factors (such as insulin dose reduction or missed insulin doses, infection, reduced caloric intake, ketogenic diet, surgery, dehydration, and alcohol abuse) and symptoms of ketoacidosis (including nausea, vomiting, abdominal pain, tiredness, and labored breathing). Inform patients that blood glucose may be normal even in the presence of ketoacidosis.
Advise patients that they may be asked to monitor ketones. If symptoms of ketoacidosis occur, instruct patients to discontinue XIGDUO XR and seek medical attention immediately [see Warnings and Precautions (5.2)].
Volume Depletion
Inform patients that symptomatic hypotension may occur with XIGDUO XR and advise them to contact their healthcare provider if they experience such symptoms [see Warnings and Precautions (5.3)]. Inform patients that dehydration may increase the risk for hypotension, and to have adequate fluid intake.
Serious Urinary Tract Infections
Inform patients of the potential for urinary tract infections, which may be serious. Provide them with information on the symptoms of urinary tract infections. Advise them to seek medical advice promptly if such symptoms occur [see Warnings and Precautions (5.4)].
Hypoglycemia with Concomitant Use of Insulin or Insulin Secretagogues
Inform patients that the incidence of hypoglycemia may increase when XIGDUO XR is added to an insulin secretagogue (e.g., sulfonylurea) and/or insulin. Educate patients on the signs and symptoms of hypoglycemia [see Warnings and Precautions (5.5)].
Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene)
Inform patients that necrotizing infections of the perineum (Fournier’s Gangrene) have occurred with dapagliflozin, a component of XIGDUO XR. Counsel patients to promptly seek medical attention if they develop pain or tenderness, redness, or swelling of the genitals or the area from the genitals back to the rectum, along with a fever above 100.4°F or malaise [see Warnings and Precautions (5.6)].
Genital Mycotic Infections in Females (e.g., Vulvovaginitis)
Inform female patients that vaginal yeast infections may occur and provide them with information on the signs and symptoms of vaginal yeast infections. Advise them of treatment options and when to seek medical advice [see Warnings and Precautions (5.8)].
Genital Mycotic Infections in Males (e.g., Balanitis or Balanoposthitis)
Inform male patients that yeast infections of the penis (e.g., balanitis or balanoposthitis) may occur, especially in patients with prior history. Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). Advise them of treatment options and when to seek medical advice [see Warnings and Precautions (5.8)].
Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions (e.g., urticaria, anaphylactic reactions, and angioedema) have been reported with the components of XIGDUO XR. Advise patients to immediately report any signs or symptoms suggesting allergic reaction or angioedema, and to take no more of the drug until they have consulted prescribing physicians.
Pregnancy
Advise pregnant patients of the potential risk to a fetus with treatment with XIGDUO XR. Instruct patients to immediately inform their healthcare provider if pregnant or planning to become pregnant [see Use in Specific Populations (8.1)].
Lactation
Advise patients that use of XIGDUO XR is not recommended while breastfeeding [see Use in Specific Populations (8.2)].
Females and Males of Reproductive Potential
Inform female patients that treatment with metformin may result in an unintended pregnancy in some premenopausal anovulatory females due to its effect on ovulation [see Use in Specific Populations (8.3)].
Administration
Instruct patients that XIGDUO XR must be swallowed whole and not crushed or chewed, and that the inactive ingredients may occasionally be eliminated in the feces as a soft mass that may resemble the original tablet.
Laboratory Tests
Due to the mechanism of action of dapagliflozin, patients taking XIGDUO XR will test positive for glucose in their urine.
Missed Dose
If a dose is missed, advise patients to take it as soon as it is remembered unless it is almost time for the next dose, in which case patients should skip the missed dose and take the medicine at the next regularly scheduled time. Advise patients not to take two doses of XIGDUO XR at the same time.
GLUCOPHAGE® is a registered trademark of Merck Santé S.A.S., a subsidiary of Merck KGaA of Darmstadt, Germany, licensed to Bristol-Myers Squibb Company.
FARXIGA® is a registered trademark of the AstraZeneca group of companies.
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
OVERDOSAGE SECTION
10 OVERDOSAGE
Dapagliflozin
In the event of an overdose, contact the Poison Control Center. The removal of dapagliflozin by hemodialysis has not been studied.
Metformin HCl
Overdose of metformin HCl has occurred, including ingestion of amounts >50 grams. Lactic acidosis has been reported in approximately 32% of metformin overdose cases [see Warnings and Precautions (5.1)]. Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.
SPL MEDGUIDE SECTION
MEDICATION GUIDE
|
MEDICATION GUIDE XIGDUO**®**** XR [ZIG-DO-OH X-R]** (dapagliflozin and metformin hydrochloride extended-release) tablets, for oral use | ||
|
What is the most important information I should know about XIGDUO XR? XIGDUO XR can cause serious side effects, including: • Stop taking XIGDUO XR and call your healthcare provider right away if you have any of the following symptoms, which could be signs of lactic acidosis: o o o o o o o o Most people who have had lactic acidosis with metformin have other things that, combined with the metformin use, led to the lactic acidosis. Tell your healthcare provider if you have any of the following, because you have a higher chance for getting lactic acidosis with XIGDUO XR if you: o o o o o o o o The best way to keep from having a problem with lactic acidosis from metformin is to tell your healthcare provider if you have any of the problems in the list above. Your healthcare provider may decide to stop your XIGDUO XR for a while if you have any of these things. • | ||
|
o o o |
o o o | |
|
XIGDUO XR can have other serious side effects. See “What are the possible side effects of XIGDUO XR?” | ||
|
What is XIGDUO XR? • o o o o • • • • | ||
|
Who should not take XIGDUO XR? Do not take XIGDUO XR if you: • • o o o • | ||
|
What should I tell my healthcare provider before taking XIGDUO XR? Before you take XIGDUO XR, tell your healthcare provider if you: • • • • • • • • • • • • • • • • • • • • Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. XIGDUO XR may affect the way other medicines work and other medicines may affect the way XIGDUO XR works. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine. | ||
|
How should I take XIGDUO XR? • • • • • • • • • • • | ||
|
What should I avoid while taking XIGDUO XR? • | ||
|
What are the possible side effects of XIGDUO XR? XIGDUO XR may cause serious side effects including: See “What is the most important information I should know about XIGDUO XR?”. • o o o o • • | ||
|
∘ ∘ ∘ ∘ |
∘ ∘ ∘ |
∘ ∘ ∘ |
|
• | ||
|
∘ |
∘ |
∘ |
|
• • • ∘ ∘ ∘ • ∘ ∘ ∘ ∘ The most common side effects of XIGDUO XR include: | ||
|
∘ ∘ ∘ |
∘ ∘ | |
|
Tell your healthcare provider or pharmacist if you have any side effect that bothers you or does not go away. These are not all of the possible side effects of XIGDUO XR. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||
|
How should I store XIGDUO XR? Store XIGDUO XR at room temperature between 68°F and 77°F (20°C and 25°C). Keep XIGDUO XR and all medicines out of the reach of children. | ||
|
General information about the safe and effective use of XIGDUO XR. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use XIGDUO XR for a condition for which it is not prescribed. Do not give XIGDUO XR to other people, even if they have the same symptoms you have. It may harm them. This Medication Guide summarizes the most important information about XIGDUO XR. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about XIGDUO XR that is written for health professionals. For more information, go to www.xigduoxr.com or call 1-800-236-9933 | ||
|
What are the ingredients in XIGDUO XR? Active ingredients: dapagliflozin and metformin hydrochloride Inactive ingredients: microcrystalline cellulose, lactose anhydrous, crospovidone, silicon dioxide, magnesium stearate, carboxymethylcellulose sodium, and hypromellose. The film coatings contain the following inactive ingredients: polyvinyl alcohol, titanium dioxide, polyethylene glycol, and talc. Additionally, the film coating for the XIGDUO XR 5 mg/500 mg tablets contains FD&C Yellow No. 6/Sunset Yellow FCF aluminum lake and the film coating for the XIGDUO XR 2.5 mg/1000 mg, 5 mg/1000 mg, 10 mg/500 mg, and 10 mg/1000 mg tablets contains iron oxides. | ||
|
Distributed by: AstraZeneca Pharmaceuticals LP Wilmington, DE 19850 |
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 09/2023
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data showing adverse renal effects, XIGDUO XR is not recommended during the second and third trimesters of pregnancy.
Limited data with XIGDUO XR or dapagliflozin in pregnant women are not sufficient to determine drug-associated risk for major birth defects or miscarriage. Published studies with metformin use during pregnancy have not reported a clear association with metformin and major birth defect or miscarriage risk (see Data). There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations).
In animal studies, adverse renal pelvic and tubule dilatations, that were not fully reversible, were observed in rats when dapagliflozin was administered during a period of renal development corresponding to the late second and third trimesters of human pregnancy, at all doses tested; the lowest of which provided an exposure 15-times the 10 mg clinical dose (see Data).
The estimated background risk of major birth defects is 6 to 10% in women with pre-gestational diabetes with a HbA1c greater than 7% and has been reported to be as high as 20 to 25% in women with HbA1c greater than 10%. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryofetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Human Data
Published data from post-marketing studies have not reported a clear association with metformin and major birth defects, miscarriage, or adverse maternal or fetal outcomes when metformin was used during pregnancy. However, these studies cannot definitely establish the absence of any metformin- associated risk because of methodological limitations, including small sample size and inconsistent comparator groups.
Animal Data
Dapagliflozin
Dapagliflozin dosed directly to juvenile rats from postnatal day (PND) 21 until PND 90 at doses of 1, 15, or 75 mg/kg/day, increased kidney weights and increased the incidence of renal pelvic and tubular dilatations at all dose levels. Exposure at the lowest dose tested was 15-times the 10 mg clinical dose (based on AUC). The renal pelvic and tubular dilatations observed in juvenile animals did not fully reverse within a 1-month recovery period.
In a prenatal and postnatal development study, dapagliflozin was administered to maternal rats from gestation day 6 through lactation day 21 at doses of 1, 15, or 75 mg/kg/day, and pups were indirectly exposed in utero and throughout lactation. Increased incidence or severity of renal pelvic dilatation was observed in 21-day-old pups offspring of treated dams at 75 mg/kg/day (maternal and pup dapagliflozin exposures were 1415-times and 137-times, respectively, the human values at the 10 mg clinical dose, based on AUC). Dose-related reductions in pup body weights were observed at greater or equal to 29-times the 10 mg clinical dose (based on AUC). No adverse effects on developmental endpoints were noted at 1 mg/kg/day (19-times the 10 mg clinical dose, based on AUC). These outcomes occurred with drug exposure during periods of renal development in rats that corresponds to the late second and third trimester of human development.
In embryofetal development studies in rats and rabbits, dapagliflozin was administered throughout organogenesis, corresponding to the first trimester of human pregnancy. In rats, dapagliflozin was neither embryolethal nor teratogenic at doses up to 75 mg/kg/day (1441-times the 10 mg clinical dose, based on AUC). Dose related effects on the rat fetus (structural abnormalities and reduced body weight) occurred only at higher dosages, equal to or greater than 150 mg/kg (more than 2344-times the 10 mg clinical dose, based on AUC), which were associated with maternal toxicity. No developmental toxicities were observed in rabbits at doses up to 180 mg/kg/day (1191-times the 10 mg clinical dose, based on AUC).
Metformin HCl
Metformin HCl did not cause adverse developmental effects when administered to pregnant Sprague Dawley rats and rabbits up to 600 mg/kg/day during the period of organogenesis. This represents an exposure of about 2- and 6-times a 2000 mg clinical dose based on body surface area (mg/m2) for rats and rabbits, respectively. Determination of fetal concentrations demonstrated a partial placental barrier to metformin.
8.2 Lactation
Risk Summary
There is no information regarding the presence of XIGDUO XR or dapagliflozin in human milk, the effects on the breastfed infant, or the effects on milk production.
Limited published studies report that metformin is present in human milk (see Data). However, there is insufficient information on the effects of metformin on the breastfed infant and no available information on the effects of metformin on milk production. Dapagliflozin is present in the milk of lactating rats (see Data). However, due to species specific differences in lactation physiology, the clinical relevance of these data is not clear. Since human kidney maturation occurs in utero and during the first 2 years of life when lactational exposure may occur, there may be risk to the developing human kidney.
Because of the potential for serious adverse reactions in breastfed infants, advise women that use of XIGDUO XR is not recommended while breastfeeding.
Data
Dapagliflozin
Dapagliflozin was present in rat milk at a milk/plasma ratio of 0.49, indicating that dapagliflozin and its metabolites are transferred into milk at a concentration that is approximately 50% of that in maternal plasma. Juvenile rats directly exposed to dapagliflozin showed risk to the developing kidney (renal pelvic and tubular dilatations) during maturation.
Metformin HCl
Published clinical lactation studies report that metformin is present in human milk which resulted in infant doses approximately 0.11% to 1% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 0.13 and 1. However, the studies were not designed to definitely establish the risk of use of metformin during lactation because of small sample size and limited adverse event data collected in infants.
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as therapy with metformin may result in ovulation in some anovulatory women.
8.4 Pediatric Use
Safety and effectiveness of XIGDUO XR in pediatric patients under 18 years of age have not been established.
8.5 Geriatric Use
XIGDUO XR
No XIGDUO XR dosage change is recommended based on age. More frequent assessment of renal function is recommended in elderly patients.
Dapagliflozin
A total of 1424 (24%) of the 5936 dapagliflozin-treated patients were 65 years and older and 207 (3.5%) patients were 75 years and older in a pool of 21 double-blind, controlled, clinical studies assessing the efficacy of dapagliflozin in improving glycemic control. After controlling for level of renal function (eGFR), efficacy was similar for patients under age 65 years and those 65 years and older. In patients ≥65 years of age, a higher proportion of patients treated with dapagliflozin for glycemic control had adverse reactions of hypotension [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
In both the DAPA-HF and DAPA-CKD studies, safety and efficacy were similar for patients age 65 years and younger and those older than 65 in both the overall population and the patients with type 2 diabetes mellitus. In the DAPA-HF study, 2714 (57%) out of 4744 patients with heart failure with reduced ejection fraction (HFrEF) were older than 65 years. Out of 2139 patients with HFrEF and type 2 diabetes mellitus, 1211 (57%) were older than 65 years. In the DAPA-CKD study, 1818 (42%) out of 4304 patients with chronic kidney disease were older than 65 years. Out of 2906 patients with chronic kidney disease and type 2 diabetes mellitus, 1399 (48%) were older than 65 years.
Metformin HCl
Controlled clinical studies of metformin did not include sufficient numbers of elderly patients to determine whether they respond differently than younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy and the higher risk of lactic acidosis. Assess renal function more frequently in elderly patients [see Warnings and Precautions (5.1)].
8.6 Renal Impairment
Initiation of XIGDUO XR is not recommended in patients with an eGFR below 45 mL/min/1.73 m2 and is contraindicated in patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2), end-stage renal disease or patients on dialysis [see Dosage and Administration (2.4), Contraindications (4) and Warnings and Precautions (5.1, 5.3)].
Dapagliflozin
Dapagliflozin 10 mg was evaluated in 4304 patients with chronic kidney disease (eGFR 25 to 75 mL/min/1.73 m2) in the DAPA-CKD study. Dapagliflozin 10 mg was also evaluated in 1926 patients with an eGFR of 30 to 60 mL/min/1.73 m2 in the DAPA-HF study. The safety profile of dapagliflozin across eGFR subgroups was consistent with the known safety profile [see Adverse Reactions (6.1) and Clinical Studies (14.3 and 14.4)].
Dapagliflozin 10 mg was evaluated in two glycemic control studies that included patients with moderate renal impairment (an eGFR of 45 to less than 60 mL/min/1.73 m2, and an eGFR of 30 to less than 60 mL/min/1.73 m2) [see Clinical Studies (14.1)]. Patients with diabetes and renal impairment using dapagliflozin 10 mg are more likely to experience hypotension and may be at higher risk for acute kidney injury secondary to volume depletion. In the study of patients with an eGFR 30 to less than 60 mL/min/1.73 m2, 13 patients receiving dapagliflozin experienced bone fractures compared to none receiving placebo. Use of dapagliflozin 10 mg for glycemic control in patients without established CV disease or CV risk factors is not recommended when eGFR is less than 45 mL/min/1.73 m2 [see Dosage and Administration (2.4)].
Metformin HCl
Metformin is substantially excreted by the kidney, and the risk of metformin accumulation and lactic acidosis increases with the degree of renal impairment. XIGDUO XR is contraindicated in severe renal impairment, patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2 [see Dosage and Administration (2.4), Contraindications (4), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Use of metformin in patients with hepatic impairment has been associated with some cases of lactic acidosis. XIGDUO XR is not recommended in patients with hepatic impairment [see Warnings and Precautions (5.1)].
•
Pregnancy: Advise females of the potential risk to a fetus, especially during the second and third trimesters. (8.1)
•
Lactation: Not recommended when breastfeeding. (8.2)
•
Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy. (8.3)
•
Geriatrics: Higher incidence of adverse reactions related to hypotension. Assess renal function more frequently. (8.5, 8.6)
•
Renal Impairment: Higher incidence of adverse reactions related to volume depletion. (8.6)
•
Hepatic Impairment: Avoid use in patients with hepatic impairment. (8.7)
DESCRIPTION SECTION
11 DESCRIPTION
XIGDUO XR tablets contain: dapagliflozin, a SGLT2 inhibitor, and metformin HCl, a biguanide.
Dapagliflozin
Dapagliflozin is described chemically as D-glucitol, 1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-, (1S)-, compounded with (2S)-1,2-propanediol, hydrate (1:1:1). The empirical formula is C21H25ClO6•C3H8O2•H2O and the formula weight is 502.98. The structural formula is:
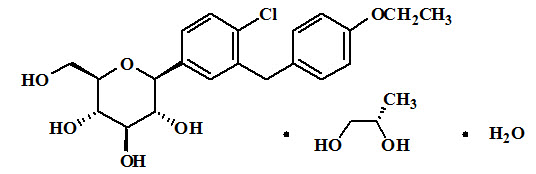
Metformin hydrochloride
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is a white to off-white crystalline compound with a molecular formula of C4H11N5•HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water, slightly soluble in alcohol, and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is:
XIGDUO XR
XIGDUO XR is available for oral administration as tablets containing the equivalent of 2.5 mg dapagliflozin as dapagliflozin propanediol and 1000 mg metformin hydrochloride which is equivalent to 779.86 mg metformin base (XIGDUO XR 2.5 mg/1,000 mg), 5 mg dapagliflozin as dapagliflozin propanediol and 500 mg metformin hydrochloride which is equivalent to 389.9 mg metformin base (XIGDUO XR 5 mg/500 mg), the equivalent of 5 mg dapagliflozin as dapagliflozin propanediol and 1,000 mg metformin hydrochloride which is equivalent to 779.86 mg metformin base (XIGDUO XR 5 mg/1,000 mg), the equivalent of 10 mg dapagliflozin as dapagliflozin propanediol and 500 mg metformin hydrochloride which is equivalent to 389.9 mg metformin base (XIGDUO XR 10 mg/500 mg), or the equivalent of 10 mg dapagliflozin as dapagliflozin propanediol and 1,000 mg metformin hydrochloride which is equivalent to 779.86 mg metformin base (XIGDUO XR 10 mg/1,000 mg).
Each film-coated tablet of XIGDUO XR contains the following inactive ingredients: microcrystalline cellulose, lactose anhydrous, crospovidone, silicon dioxide, magnesium stearate, carboxymethylcellulose sodium, and hypromellose.
The film coatings contain the following inactive ingredients: polyvinyl alcohol, titanium dioxide, polyethylene glycol, and talc. Additionally, the film coating for the XIGDUO XR 5 mg/500 mg tablets contains FD&C Yellow No. 6/Sunset Yellow FCF aluminum lake. The film coating for the XIGDUO XR 2.5 mg/1,000 mg, 5 mg/1000 mg, 10 mg/500 mg, and 10 mg/1,000 mg tablets contains iron oxides.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dapagliflozin
Sodium-glucose cotransporter 2 (SGLT2), expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen. Dapagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose, and thereby promotes urinary glucose excretion. Dapagliflozin also reduces sodium reabsorption and increases the delivery of sodium to the distal tubule. This may influence several physiological functions including, but not restricted to, lowering both pre- and afterload of the heart and downregulation of sympathetic activity, and decreased intraglomerular pressure which is believed to be mediated by increased tubuloglomerular feedback.
Metformin HCl
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may decrease.
12.2 Pharmacodynamics
General
Dapagliflozin
Increases in the amount of glucose excreted in the urine were observed in healthy subjects and in patients with type 2 diabetes mellitus following the administration of dapagliflozin (see Figure 1). Dapagliflozin doses of 5 or 10 mg per day in patients with type 2 diabetes mellitus for 12 weeks resulted in excretion of approximately 70 grams of glucose in the urine per day. A near maximum glucose excretion was observed at the dapagliflozin daily dose of 20 mg. This urinary glucose excretion with dapagliflozin also results in increases in urinary volume [see Adverse Reactions (6.1)]. After discontinuation of dapagliflozin, on average, the elevation in urinary glucose excretion approaches baseline by about 3 days for the 10 mg dose.
Figure 1: Scatter Plot and Fitted Line of Change from Baseline in 24-Hour Urinary Glucose Amount versus Dapagliflozin Dose in Healthy Subjects and Subjects with Type 2 Diabetes Mellitus (T2DM) (Semi-Log Plot)
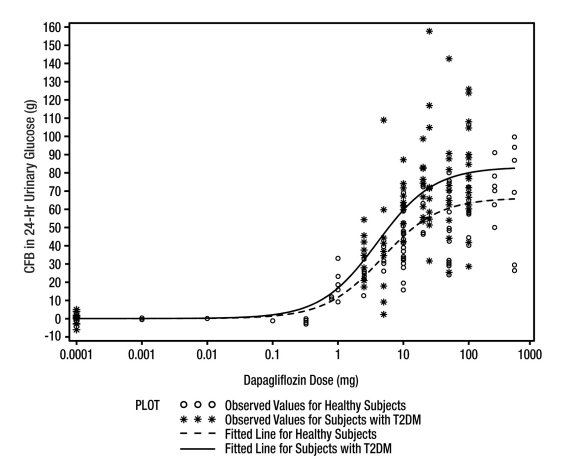
Cardiac Electrophysiology
Dapagliflozin was not associated with clinically meaningful prolongation of QTc interval at daily doses up to 150 mg (15-times the recommended maximum dose) in a study of healthy subjects. In addition, no clinically meaningful effect on QTc interval was observed following single doses of up to 500 mg (50-times the recommended maximum dose) of dapagliflozin in healthy subjects.
12.3 Pharmacokinetics
XIGDUO XR
The administration of XIGDUO XR in healthy subjects after a standard meal compared to the fasted state resulted in the same extent of exposure for both dapagliflozin and metformin extended-release. Compared to the fasted state, the standard meal resulted in 35% reduction and a delay of 1 to 2 hours in the peak plasma concentrations of dapagliflozin. This effect of food is not considered to be clinically meaningful. Food has no relevant effect on the pharmacokinetics of metformin when administered as XIGDUO XR combination tablets.
Absorption
Dapagliflozin
Following oral administration of dapagliflozin, the maximum plasma concentration (Cmax) is usually attained within 2 hours under fasting state. The Cmax and AUC values increase dose proportionally with increase in dapagliflozin dose in the therapeutic dose range. The absolute oral bioavailability of dapagliflozin following the administration of a 10 mg dose is 78%. Administration of dapagliflozin with a high-fat meal decreases its Cmax by up to 50% and prolongs Tmax by approximately 1 hour, but does not alter AUC as compared with the fasted state. These changes are not considered to be clinically meaningful and dapagliflozin can be administered with or without food.
Metformin HCl
Following a single oral dose of metformin extended-release, Cmax is achieved with a median value of 7 hours and a range of 4 to 8 hours. The extent of metformin absorption (as measured by AUC) from the metformin extended-release tablet increased by approximately 50% when given with food. There was no effect of food on Cmax and Tmax of metformin.
Distribution
Dapagliflozin
Dapagliflozin is approximately 91% protein bound. Protein binding is not altered in patients with renal or hepatic impairment.
Metformin HCl
Distribution studies with extended-release metformin have not been conducted; however, the apparent volume of distribution (V/F) of metformin following single oral doses of immediate-release metformin 850 mg averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins, in contrast to sulfonylureas, which are more than 90% protein bound. Metformin partitions into erythrocytes.
Metabolism
Dapagliflozin
The metabolism of dapagliflozin is primarily mediated by UGT1A9; CYP-mediated metabolism is a minor clearance pathway in humans. Dapagliflozin is extensively metabolized, primarily to yield dapagliflozin 3-O-glucuronide, which is an inactive metabolite. Dapagliflozin 3-O-glucuronide accounted for 61% of a 50 mg [14C]-dapagliflozin dose and is the predominant drug-related component in human plasma.
Metformin HCl
Intravenous single-dose studies in healthy subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) or biliary excretion.
Metabolism studies with extended-release metformin tablets have not been conducted.
Elimination
Dapagliflozin
Dapagliflozin and related metabolites are primarily eliminated via the renal pathway. Following a single 50 mg dose of [14C]-dapagliflozin, 75% and 21% total radioactivity is excreted in urine and feces, respectively. In urine, less than 2% of the dose is excreted as parent drug. In feces, approximately 15% of the dose is excreted as parent drug. The mean plasma terminal half-life (t½) for dapagliflozin is approximately 12.9 hours following a single oral dose of dapagliflozin 10 mg.
Metformin HCl
Renal clearance is approximately 3.5-times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Specific Populations
Renal Impairment
Dapagliflozin
At steady-state (20 mg once daily dapagliflozin for 7 days), patients with type 2 diabetes mellitus with mild, moderate, or severe renal impairment (as determined by eGFR) had geometric mean systemic exposures of dapagliflozin that were 45%, 100% and 200% higher, respectively, as compared to patients with type 2 diabetes mellitus with normal renal function. Higher systemic exposure of dapagliflozin in patients with type 2 diabetes mellitus with renal impairment did not result in a correspondingly higher 24-hour urinary glucose excretion. The steady-state 24-hour urinary glucose excretion in patients with type 2 diabetes mellitus and mild, moderate, and severe renal impairment was 42%, 80%, and 90% lower, respectively, than in patients with type 2 diabetes mellitus with normal renal function.
The impact of hemodialysis on dapagliflozin exposure is not known [see Dosage and Administration (2.4), Warnings and Precautions (5.3), Use in Specific Populations (8.6) and Clinical Studies (14)].
Metformin HCl
In patients with decreased renal function, the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased [see Contraindications (4) and Warnings and Precautions (5.1)].
Hepatic Impairment
Dapagliflozin
In patients with mild and moderate hepatic impairment (Child-Pugh classes A and B), mean Cmax and AUC of dapagliflozin were up to 12% and 36% higher, respectively, as compared to healthy matched control subjects following single-dose administration of 10 mg dapagliflozin. These differences were not considered to be clinically meaningful. In patients with severe hepatic impairment (Child-Pugh class C), mean Cmax and AUC of dapagliflozin were up to 40% and 67% higher, respectively, as compared to healthy matched controls.
Metformin HCl
No pharmacokinetic studies of metformin have been conducted in patients with hepatic impairment [see Warnings and Precautions (5.1)].
Geriatric
Dapagliflozin
Based on a population pharmacokinetic analysis, age does not have a clinically meaningful effect on systemic exposures of dapagliflozin.
Metformin HCl
Limited data from controlled pharmacokinetic studies of metformin in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and Cmax is increased, compared to healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function.
Gender
Dapagliflozin
Based on a population pharmacokinetic analysis, gender does not have a clinically meaningful effect on systemic exposures of dapagliflozin.
Metformin HCl
Metformin pharmacokinetic parameters did not differ significantly between healthy subjects and patients with type 2 diabetes mellitus when analyzed according to gender (males=19, females=16). Similarly, in controlled clinical studies in patients with type 2 diabetes, the antihyperglycemic effect of metformin was comparable in males and females.
Race
Dapagliflozin
Based on a population pharmacokinetic analysis, race (White, Black, or Asian) does not have a clinically meaningful effect on systemic exposures of dapagliflozin.
Metformin HCl
No studies of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical studies of metformin in patients with type 2 diabetes mellitus, the antihyperglycemic effect was comparable in Whites (n=249), Blacks (n=51), and Hispanics (n=24).
Body Weight
Dapagliflozin
Based on a population pharmacokinetic analysis, body weight does not have a clinically meaningful effect on systemic exposures of dapagliflozin.
Drug Interactions
Specific pharmacokinetic drug interaction studies with XIGDUO XR have not been performed, although such studies have been conducted with the individual dapagliflozin and metformin components.
In Vitro Assessment of Drug Interactions
Dapagliflozin
In in vitro studies, dapagliflozin and dapagliflozin 3-O-glucuronide neither inhibited CYP 1A2, 2C9, 2C19, 2D6, or 3A4, nor induced CYP 1A2, 2B6, or 3A4. Dapagliflozin is a weak substrate of the P-glycoprotein (P‑gp) active transporter, and dapagliflozin 3-O-glucuronide is a substrate for the OAT3 active transporter. Dapagliflozin or dapagliflozin 3-O-glucuronide did not meaningfully inhibit P-gp, OCT2, OAT1, or OAT3 active transporters. Overall, dapagliflozin is unlikely to affect the pharmacokinetics of concurrently administered medications that are P-gp, OCT2, OAT1, or OAT3 substrates.
Effects of Other Drugs on Metformin
Table 7 shows the effect of other coadministered drugs on metformin.
Table 7: Effect of Coadministered Drug on Plasma Metformin Systemic Exposure
| |||
|
Coadministered Drug (Dose Regimen)* |
Metformin (Dose Regimen)* |
Metformin | |
|
Change†** in AUC**‡ |
Change†** in C****max** | ||
|
No dosing adjustments required for the following: | |||
|
Glyburide (5 mg) |
850 mg |
↓9%§ |
↓7%§ |
|
Furosemide (40 mg) |
850 mg |
↑15%§ |
↑22%§ |
|
Nifedipine (10 mg) |
850 mg |
↑9% |
↑20% |
|
Propranolol (40 mg) |
850 mg |
↓10% |
↓6% |
|
Ibuprofen (400 mg) |
850 mg |
↑5%§ |
↑7%§ |
|
Drugs eliminated by renal tubular secretion may increase the accumulation of metformin[see Drug Interactions (7)]. | |||
|
Cimetidine (400 mg) |
850 mg |
↑40% |
↑60% |
Effects of Metformin on Other Drugs
Table 8 shows the effect of metformin on other coadministered drugs.
Table 8: Effect of Metformin on Coadministered Drug Systemic Exposure|
Coadministered Drug |
Metformin |
Coadministered Drug | |
|---|---|---|---|
|
Change†** in AUC**‡ |
Change†** in C****max** | ||
| |||
|
No dosing adjustments required for the following: | |||
|
Glyburide (5 mg) |
850 mg |
↓22%§ |
↓37%§ |
|
Furosemide (40 mg) |
850 mg |
↓12%§ |
↓31%§ |
|
Nifedipine (10 mg) |
850 mg |
↑10%¶ |
↑8% |
|
Propranolol (40 mg) |
850 mg |
↑1%¶ |
↑2% |
|
Ibuprofen (400 mg) |
850 mg |
↓3%# |
↑1%# |
|
Cimetidine (400 mg) |
850 mg |
↓5%¶ |
↑1% |
Effects of Other Drugs on Dapagliflozin
Table 9 shows the effect of coadministered drugs on dapagliflozin. No dose adjustments are recommended for dapagliflozin.
Table 9: Effects of Coadministered Drugs on Dapagliflozin Systemic Exposure
| |||
|
Coadministered Drug |
Dapagliflozin |
Dapagliflozin | |
|
Change†** in AUC**‡ |
Change†** in C****max** | ||
|
No dosing adjustments required for the following: | |||
|
Oral Antidiabetic Agents | |||
|
Metformin (1000 mg) |
20 mg |
↓1% |
↓7% |
|
Pioglitazone (45 mg) |
50 mg |
0% |
↑9% |
|
Sitagliptin (100 mg) |
20 mg |
↑8% |
↓4% |
|
Glimepiride (4 mg) |
20 mg |
↓1% |
↑1% |
|
Voglibose (0.2 mg three times daily) |
10 mg |
↑1% |
↑4% |
|
Other Medications | |||
|
Hydrochlorothiazide (25 mg) |
50 mg |
↑7% |
↓1% |
|
Bumetanide (1 mg) |
10 mg once daily |
↑5% |
↑8% |
|
Valsartan (320 mg) |
20 mg |
↑2% |
↓12% |
|
Simvastatin (40 mg) |
20 mg |
↓1% |
↓2% |
|
Anti-infective Agent | |||
|
Rifampin (600 mg once daily for 6 days) |
10 mg |
↓22% |
↓7% |
|
Nonsteroidal Anti-inflammatory Agent | |||
|
Mefenamic Acid (loading dose of 500 mg followed by 14 doses of 250 mg every 6 hours) |
10 mg |
↑51% |
↑13% |
Effects of Dapagliflozin on Other Drugs
Table 10 shows the effect of dapagliflozin on other coadministered drugs. Dapagliflozin did not meaningfully affect the pharmacokinetics of the coadministered drugs.
Table 10: Effects of Dapagliflozin on the Systemic Exposures of Coadministered Drugs
| |||
|
Coadministered Drug |
Dapagliflozin |
Coadministered Drug | |
|
Change†****in AUC‡ |
Change†** in C****max** | ||
|
No dosing adjustments required for the following: | |||
|
Oral Antidiabetic Agents | |||
|
Metformin (1000 mg) |
20 mg |
0% |
↓5% |
|
Pioglitazone (45 mg) |
50 mg |
0% |
↓7% |
|
Sitagliptin (100 mg) |
20 mg |
↑1% |
↓11% |
|
Glimepiride (4 mg) |
20 mg |
↑13% |
↑4% |
|
Other Medications | |||
|
Hydrochlorothiazide (25 mg) |
50 mg |
↓1% |
↓5% |
|
Bumetanide (1 mg) |
10 mg once daily |
↑13% |
↑13% |
|
Valsartan (320 mg) |
20 mg |
↑5% |
↓6% |
|
Simvastatin (40 mg) |
20 mg |
↑19% |
↓6% |
|
Digoxin (0.25 mg) |
20 mg loading dose then 10 mg once daily for 7 days |
0% |
↓1% |
|
Warfarin (25 mg) S-warfarin |
20 mg loading dose then 10 mg once daily for 7 days |
↑3% |
↑7% |
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
XIGDUO XR
No animal studies have been conducted with XIGDUO XR to evaluate carcinogenesis, mutagenesis, or impairment of fertility. The following data are based on the findings in the studies with dapagliflozin and metformin individually.
Dapagliflozin
Dapagliflozin did not induce tumors in either mice or rats at any of the doses evaluated in 2-year carcinogenicity studies. Oral doses in mice consisted of 5, 15, and 40 mg/kg/day in males and 2, 10 and 20 mg/kg/day in females, and oral doses in rats were 0.5, 2, and 10 mg/kg/day for both males and females. The highest doses evaluated in mice were approximately 72-times (males) and 105-times (females) the clinical dose of 10 mg per day, based on AUC exposure. In rats, the highest dose was approximately 131-times (males) and 186-times (females) the clinical dose of 10 mg per day, based on AUC exposure.
Dapagliflozin was negative in the Ames mutagenicity assay and was positive in a series of in vitro clastogenicity assays in the presence of S9 activation and at concentrations greater than or equal to 100 μg/mL. Dapagliflozin was negative for clastogenicity in a series of in vivo studies evaluating micronuclei or DNA repair in rats at exposure multiples greater than 2100-times the clinical dose.
Dapagliflozin had no effects on mating, fertility, or early embryonic development in treated male or female rats at exposure multiples less than or equal to 1708-times and 998-times the maximum recommended human dose in males and females, respectively.
Metformin HCl
Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 and 1500 mg/kg/day, respectively. These doses are both approximately 4-times the maximum recommended human dose of 2000 mg based on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
There was no evidence of a mutagenic potential of metformin in the following in vitro tests: Ames test (S. typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative.
Fertility of male or female rats was unaffected by metformin when administered at doses as high as 600 mg/kg/day, which is approximately 3-times the maximum recommended human dose based on body surface area comparisons.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
XIGDUO® XR (dapagliflozin and metformin HCl extended-release) tablets have markings on one side, are plain on the reverse side, and are available in the strengths and packages listed in Table 19.
Table 19: XIGDUO XR Tablet Presentations|
Tablet Strength |
Film-Coated Tablet |
Tablet |
Pack Size |
NDC Code |
|---|---|---|---|---|
|
2.5 mg/ 1,000 mg |
Light brown to brown, biconvex, oval-shaped |
"1074" and "2.5/1000" debossed on one side and plain on the reverse side |
Bottle of 60 |
0310-6225-60 |
|
5 mg/ 500 mg |
orange, biconvex, capsule-shaped |
"1070" and "5/500" debossed on one side and plain on the reverse side |
Bottle of 30 |
0310-6250-30 0310-6250-50 |
|
5 mg/ 1,000 mg |
pink to dark pink, biconvex, oval-shaped |
"1071" and "5/1000" debossed on one side and plain on the reverse side |
Bottle of 30 |
0310-6260-30 0310-6260-60 0310-6260-90 0310-6260-40 |
|
10 mg/ 500 mg |
pink, biconvex, capsule-shaped |
"1072" and "10/500" debossed on one side and plain on the reverse side |
Bottle of 30 |
0310-6270-30 0310-6270-50 |
|
10 mg/ 1,000 mg |
yellow to dark yellow, biconvex, oval-shaped |
"1073" and "10/1000" debossed on one side and plain on the reverse side |
Bottle of 30 |
0310-6280-30 0310-6280-90 0310-6280-40 |
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
