ALLERGYCALM
AllergyCalm Pellets
deae124a-4db4-41fc-e053-2a95a90ac222
HUMAN OTC DRUG LABEL
May 12, 2025
Boiron
DUNS: 282560473
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
SCHOENOCAULON OFFICINALE SEED, ONION, SOLIDAGO VIRGAUREA FLOWERING TOP, AMBROSIA ARTEMISIIFOLIA, EUPHRASIA STRICTA, HISTAMINE DIHYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL



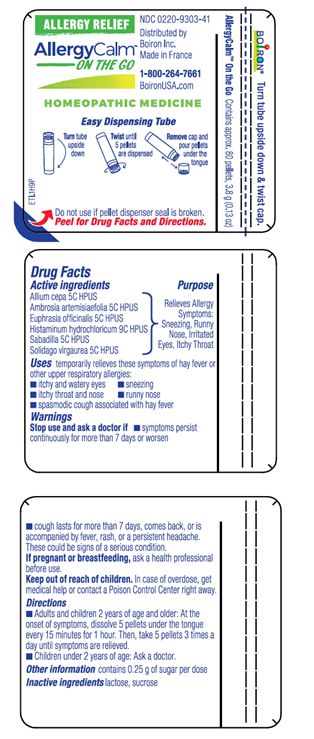
INDICATIONS & USAGE SECTION
Uses*
temporarily relieves these symptoms of hay fever or upper respiratory allergies:
- itchy and watery eyes
- itchy throat and nose
- sneezing
- runny nose
OTC - ACTIVE INGREDIENT SECTION
Active Ingredients**(in each pellet)
Allium cepa 5C HPUS (0.07 mg)
Ambrosia artemisiaefolia 5C HPUS (0.07 mg)
Euphrasia officinalis 5C HPUS (0.07 mg)
Histaminum hydrochloricum 9C HPUS (0.07 mg)
Sabadilla 5C HPUS (0.07 mg) (contains less than 10-12 mg cevadine alkaloids)
Solidago vigaurea 5C HPUS (0.07 mg)
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
OTC - PURPOSE SECTION
Purpose*
Relieves Allergy Symptoms: Sneezing, Runny Nose, Irritated Eyes, Itchy Throat
WARNINGS SECTION
OTC - ASK DOCTOR SECTION
Ask a doctor before use if you havea breathing problem or wheezing. These could be signs of a serious condition.
OTC - STOP USE SECTION
Stop use and ask a doctor ifsymptoms persist continuously for more than 7 days or worsen.
OTC - PREGNANCY OR BREAST FEEDING SECTION
**If pregnant or breast-feeding,**ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
**Keep out of reach of children.**In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
- Adults and children 2 years of age and older: At the onset of symptoms, dissolve 5 pellets under the tongue every 15 minutes for 1 hour. Then, dissolve 5 pellets under the tongue 3 times a day until symptoms are relieved.
- Children under 2 years of age: Ask a doctor.
SPL UNCLASSIFIED SECTION
- do not use if glued carton end flaps are open or if pellet dispenser seal is broken
- contains 0.25 g of sugar per dose
Multi-Symptom Allergy Relief*
Year-Round Allergy Relief*
Non-Drowsy
2 PORTABLE TUBES
APPROX. 80 PELLETS EACH
TOTAL 160 PELLETS
Contains approx. 80 pellets, 3.8 g (0.13 oz)
Turn tube upside down, twist until 5 pellets are dispensed
Remove cap and pour pellets under the tongue
Do not use if pellet dispenser seal is broken.
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
INACTIVE INGREDIENT SECTION
lactose, sucrose
OTC - QUESTIONS SECTION
1-800-264-7661
Made in France
Distributed by
Boiron, Inc.
Newtown Square, PA 19073
