Cefuroxime axetil
These highlights do not include all the information needed to use CEFUROXIME AXETIL TABLETS safely and effectively. See full prescribing information for CEFUROXIME AXETIL TABLETS. CEFUROXIME AXETIL tablets, for oral use Initial U.S. Approval: 1987
f38418a7-dd90-4f20-b747-7ac086c53015
HUMAN PRESCRIPTION DRUG LABEL
Aug 6, 2025
American Health Packaging
DUNS: 929561009
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Cefuroxime axetil
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Cefuroxime axetil
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel

CEFUROXIME AXETIL
TABLET, USP
500 mg
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious and otherwise important adverse reaction is described in greater detail in the Warnings and Precautions section of the label:
Anaphylactic Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Tablets
Multiple-Dose Dosing Regimens with 7 to 10 Days’ Duration: In multiple-dose
clinical trials, 912 subjects were treated with cefuroxime axetil (125 to 500
mg twice daily). It is noted that 125 mg twice daily is not an approved
dosage. Twenty (2.2%) subjects discontinued medication due to adverse
reactions. Seventeen (85%) of the 20 subjects who discontinued therapy did so
because of gastrointestinal disturbances, including diarrhea, nausea,
vomiting, and abdominal pain. The percentage of subjects treated with
cefuroxime axetil who discontinued study drug because of adverse reactions was
similar at daily doses of 1,000, 500, and 250 mg (2.3%, 2.1%, and 2.2%,
respectively). However, the incidence of gastrointestinal adverse reactions
increased with the higher recommended doses.
The adverse reactions in Table 5 are for subjects (n = 912) treated with cefuroxime axetil in multiple-dose clinical trials.
Table 3. Adverse Reactions (≥1%) after Multiple-dose Regimens with Cefuroxime Axetil Tablets|
Adverse Reaction |
Cefuroxime Axetil |
|
Blood and lymphatic system disorders | |
|
Eosinophilia |
1% |
|
Gastrointestinal disorders | |
|
Diarrhea |
4% |
|
Nausea/Vomiting |
3% |
|
Investigations | |
|
Transient elevation in AST |
2% |
|
Transient elevation in ALT |
2% |
|
Transient elevation in LDH |
1% |
The following adverse reactions occurred in less than 1% but greater than 0.1% of subjects (n = 912) treated with cefuroxime axetil in multiple-dose clinical trials.
Immune System Disorders: Hives, swollen tongue.
Metabolism and Nutrition Disorders: Anorexia.
Nervous System Disorders: Headache.
Cardiac Disorders: Chest pain.
Respiratory Disorders: Shortness of breath.
Gastrointestinal Disorders: Abdominal pain, abdominal cramps, flatulence,
indigestion, mouth ulcers.
Skin and Subcutaneous Tissue Disorders: Rash, itch
Renal and Urinary Disorders: Dysuria.
Reproductive System and Breast Disorders: Vaginitis, vulvar itch.
General Disorders and Administration Site Conditions: Chills, sleepiness,
thirst.
Investigations: Positive Coombs’ test.
Early Lyme Disease with 20-Day Regimen: Two multicenter trials assessed cefuroxime axetil tablets 500 mg twice daily for 20 days. The most common drug-related adverse experiences were diarrhea (10.6%), Jarisch-Herxheimer reaction (5.6%), and vaginitis (5.4%). Other adverse experiences occurred with frequencies comparable to those reported with 7 to 10 days’ dosing.
Single-Dose Regimen for Uncomplicated Gonorrhea: In clinical trials using a single 1,000 mg dose of cefuroxime axetil, 1,061 subjects were treated for uncomplicated gonorrhea.
The adverse reactions in Table 6 were for subjects treated with a single dose of 1,000 mg cefuroxime axetil in U.S. clinical trials.
Table 4. Adverse Reactions (≥1%) after Single-dose Regimen with 1,000-mg Cefuroxime Axetil Tablets for Uncomplicated Gonorrhea|
Adverse Reaction |
Cefuroxime axetil |
|
Gastrointestinal disorders | |
|
Nausea/Vomiting |
7% |
|
Diarrhea |
4% |
The following adverse reactions occurred in less than 1% but greater than 0.1% of subjects (n = 1,061) treated with a single dose of cefuroxime axetil 1,000 mg for uncomplicated gonorrhea in U.S. clinical trials.
Infections and Infestations: Vaginal candidiasis.
Nervous System Disorders: Headache, dizziness, somnolence.
Cardiac Disorders: Tightness/pain in chest, tachycardia.
Gastrointestinal Disorders: Abdominal pain, dyspepsia.
Skin and Subcutaneous Tissue Disorders: Erythema, rash, pruritus.
Musculoskeletal and Connective Tissue Disorders: Muscle cramps, muscle
stiffness, muscle spasm of neck, lockjaw-type reaction.
Renal and Urinary Disorders: Bleeding/pain in urethra, kidney pain.
Reproductive System and Breast Disorders: Vaginal itch, vaginal discharge.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of cefuroxime axetil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders
Hemolytic anemia, leukopenia, pancytopenia, thrombocytopenia.
Gastrointestinal Disorders
Pseudomembranous colitis [see Warnings and Precautions (5.2)].
Hepatobiliary Disorders
Hepatic impairment including hepatitis and cholestasis, jaundice.
Immune System Disorders
Anaphylaxis, serum sickness-like reaction, acute myocardial ischemia with or
without myocardial infarction may occur as part of an allergic reaction.
Investigations
Increased prothrombin time.
Nervous System Disorders
Seizure, encephalopathy.
Renal and Urinary Disorders
Renal dysfunction.
Skin and Subcutaneous Tissue Disorders
Angioedema, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal
necrolysis, urticaria.
The most common adverse reactions (≥3%) for cefuroxime axetil tablets are diarrhea, nausea/vomiting, Jarisch-Herxheimer reaction, and vaginitis (early Lyme disease). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ascend Laboratories, LLC at 1-877-272-7901 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
11 DESCRIPTION
Cefuroxime axetil tablets, USP contain cefuroxime as cefuroxime axetil. Cefuroxime axetil is a semisynthetic, cephalosporin antibacterial drug for oral administration.
The chemical name of cefuroxime axetil (1-(acetyloxy) ethyl ester of cefuroxime) is ( RS)-1 hydroxyethyl (6 R,7 R)-7-[2-(2-furyl)glyoxyl- amido]-3-(hydroxymethyl)-8-oxo-5-thia-1 azabicyclo[4.2.0]-oct-2-ene-2-carboxylate, 72-( Z)-( O-methyl-oxime), 1-acetate 3-carbamate. Its molecular formula is C 20H 22N 4O 10S, and it has a molecular weight of 510.48.
Cefuroxime axetil is in the amorphous form and has the following structural formula:
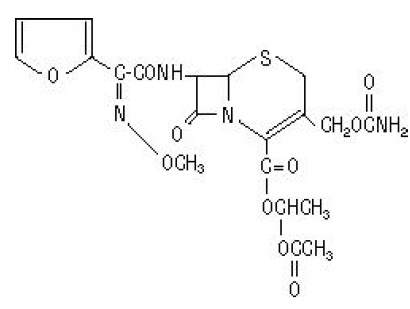
Cefuroxime axetil tablets, USP are film-coated and contain the equivalent of 250 or 500 mg of cefuroxime as cefuroxime axetil. Cefuroxime axetil tablets, USP contain the inactive ingredients microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, colloidal silicon dioxide, calcium stearate, calcium carbonate, crospovidone, hypromellose, titanium dioxide, propylene glycol, FD &C blue no.1 Aluminium lake.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Allergic Reactions
Inform patients that cefuroxime axetil is a cephalosporin that can cause
allergic reactions in some individuals [see Warnings and Precautions (5.1)].
Clostridioides difficile**-Associated Diarrhea**
Inform patients that diarrhea is a common problem caused by antibacterials,
and it usually ends when the antibacterial is discontinued. Sometimes after
starting treatment with antibacterials, patients can develop watery and bloody
stools (with or without stomach cramps and fever) even as late as 2 or more
months after having taken their last dose of the antibacterial. If this
occurs, advise patients to contact their physician as soon as possible.
Crushing Tablets
Instruct patients to swallow the tablet whole, without crushing the tablet.
Patients who cannot swallow the tablet whole should receive the oral
suspension.
Drug Resistance
Inform patients that antibacterial drugs, including cefuroxime axetil, should
only be used to treat bacterial infections. They do not treat viral infections
(e.g., the common cold). When cefuroxime axetil is prescribed to treat a
bacterial infection, inform patients that although it is common to feel better
early in the course of therapy, the medication should be taken exactly as
directed. Skipping doses or not completing the full course of therapy may: (1)
decrease the effectiveness of the immediate treatment, and (2) increase the
likelihood that bacteria will develop resistance and will not be treatable by
cefuroxime axetil or other antibacterial drugs in the future.

Manufactured by:
Alkem Laboratories Ltd.
Mumbai – 400 013, INDIA
Distributed by:
American Health Packaging
Columbus, OH 43217
Revised: May, 2025
PT 2885-06
