Losartan Potassium and Hydrochlorothiazide
These highlights do not include all the information needed to use LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE TABLETS safely and effectively. See full prescribing information for LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE TABLETS.LOSARTAN POTASSIUM and HYDROCHLOROTHIAZIDE tablets, for oral use Initial U.S. Approval: 1995
ee1eaaf6-050b-48af-942b-5e3451edc164
HUMAN PRESCRIPTION DRUG LABEL
Oct 30, 2023
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Losartan Potassium and Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Losartan Potassium and Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Losartan Potassium and Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
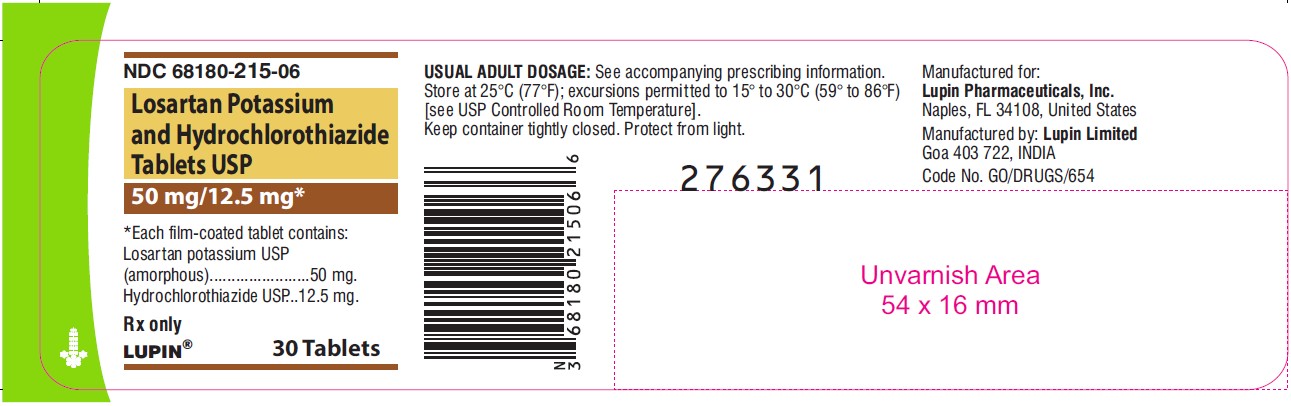
LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE TABLETS USP
Rx only
50 mg/12.5 mg
NDC 68180-215-06
30 Tablets

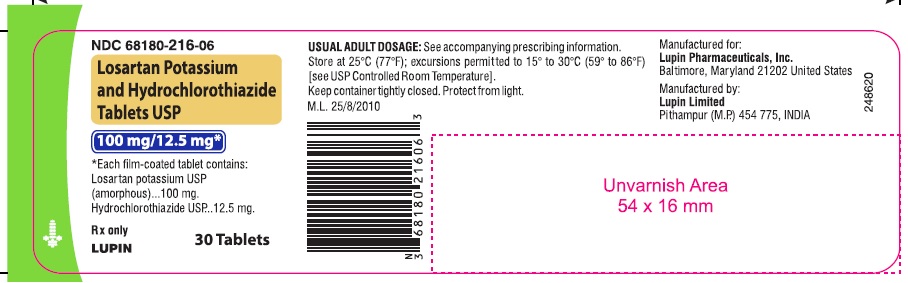
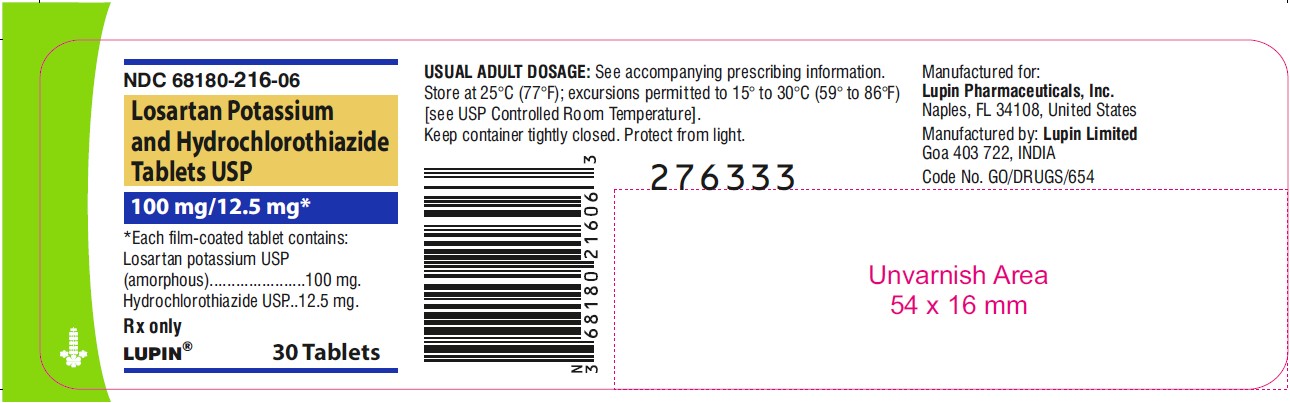
LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE TABLETS USP
Rx only
100 mg/12.5 mg
NDC 68180-216-06
30 Tablets
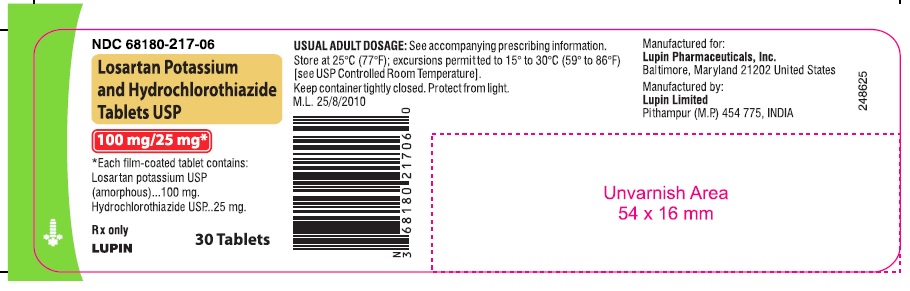
LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE TABLETS USP
Rx only
100 mg/25 mg
NDC 68180-217-06
30 Tablets

DESCRIPTION SECTION
11 DESCRIPTION
Losartan potassium and hydrochlorothiazide tablets USP, 50 mg/12.5 mg, losartan potassium and hydrochlorothiazide tablets USP, 100 mg/12.5 mg and losartan potassium and hydrochlorothiazide tablets USP, 100 mg/25 mg combine an angiotensin II receptor blocker acting on the AT1 receptor subtype and a diuretic, hydrochlorothiazide.
Losartan potassium, a non-peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-methanol monopotassium salt. Its empirical formula is C22H22ClKN6O, and its structural formula is:
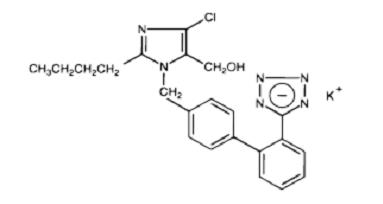
Image
Losartan potassium is a white to off-white amorphous powder with a molecular weight of 461.01. It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone.
Oxidation of the 5-hydroxymethyl group on the imidazole ring results in the active metabolite of losartan.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
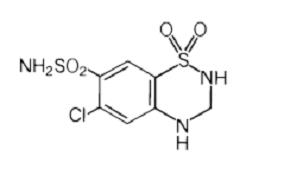
Image
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.74, which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
Losartan potassium and hydrochlorothiazide tablets USP are available for oral administration in three tablet combinations of losartan and hydrochlorothiazide. Losartan potassium and hydrochlorothiazide tablets USP, 50 mg/12.5 mg contains 50 mg of losartan potassium and 12.5 mg of hydrochlorothiazide. Losartan potassium and hydrochlorothiazide tablets USP, 100 mg/12.5 mg contains 100 mg of losartan potassium and 12.5 mg of hydrochlorothiazide. Losartan potassium and hydrochlorothiazide tablets USP, 100 mg/25 mg contains 100 mg of losartan potassium and 25 mg of hydrochlorothiazide. Inactive ingredients are colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, lactose anhydrous, magnesium stearate, microcrystalline cellulose, pregelatinized starch and titanium dioxide. Losartan potassium and hydrochlorothiazide tablets USP, 50 mg/ 12.5 mg and losartan potassium and hydrochlorothiazide tablets USP, 100 mg/ 25 mg also contain iron oxide yellow.
Losartan potassium and hydrochlorothiazide tablets USP, 50 mg/12.5 mg contain 4.24 mg (0.108 mEq) of potassium, losartan potassium and hydrochlorothiazide tablets USP, 100 mg/12.5 mg contain 8.48 mg (0.216 mEq) of potassium, and losartan potassium and hydrochlorothiazide tablets USP, 100 mg/ 25 mg contains 8.48 mg (0.216 mEq) of potassium.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Losartan potassium and hydrochlorothiazide tablet USP is supplied as a film- coated tablet.
|
** Losartan/ Hydrochlorothiazide** |
** Color** |
** Shape** |
** Engraving** |
** NDC 68180-xxx-xx** | ||
|
** One side** |
** Other side** |
** Bottle/30** |
** Bottle/90** | |||
|
50 mg/12.5 mg |
Yellow |
Capsule shaped biconvex |
LU |
M41 |
215-06 |
215-09 |
|
100 mg/12.5 mg |
White |
Oval shaped biconvex |
LU |
M42 |
216-06 |
216-09 |
|
100 mg/25 mg |
Yellow |
Tear drop shaped biconvex |
LU |
M43 |
217-06 |
217-09 |
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed. Protect from light.
