LEMTRADA
These highlights do not include all the information needed to use LEMTRADA safely and effectively. See full prescribing information for LEMTRADA. LEMTRADA (alemtuzumab) injection, for intravenous use Initial U.S. Approval: 2001
6236b0bc-82e9-4447-9a78-f57d94770269
HUMAN PRESCRIPTION DRUG LABEL
Feb 21, 2024
Genzyme Corporation
DUNS: 025322157
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ALEMTUZUMAB
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 12 mg/1.2 mL Vial Carton
NDC 58468-0200-1
LEMTRADA®
(alemtuzumab)
injection
12 mg/1.2 mL
(10 mg/mL)
For Intravenous Infusion Only
Dilute before
Intravenous Infusion
Single-Dose Vial,
Discard Unused Portion
Rx only
Dispense the enclosed
Medication Guide to each
patient.
BOXED WARNING SECTION
WARNING: AUTOIMMUNITY, INFUSION REACTIONS, STROKE, AND MALIGNANCIES
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
LEMTRADA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Because of its safety profile, the use of LEMTRADA should generally be reserved for patients who have had an inadequate response to two or more drugs indicated for the treatment of MS [see Warnings and Precautions (5)].
Limitations of Use
LEMTRADA is not recommended for use in patients with clinically isolated syndrome (CIS) because of its safety profile [see Warnings and Precautions (5)].
- LEMTRADA is a CD52-directed cytolytic monoclonal antibody indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Because of its safety profile, the use of LEMTRADA should generally be reserved for patients who have had an inadequate response to two or more drugs indicated for the treatment of MS. (1.5)
Limitations of Use:
LEMTRADA is not recommended for use in patients with clinically isolated syndrome (CIS) because of its safety profile. (1.5)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
LEMTRADA is contraindicated in patients:
- with known hypersensitivity or anaphylactic reactions to alemtuzumab or any of the excipients in LEMTRADA
- who are infected with human immunodeficiency virus (HIV) because LEMTRADA causes prolonged reductions of CD4+ lymphocyte counts
- with active infection
- Known hypersensitivity or anaphylactic reactions to alemtuzumab or any of the excipients in LEMTRADA (4)
- Infection with Human Immunodeficiency Virus (4)
- Active infection (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Autoimmunity [see Boxed Warning and Warnings and Precautions (5.1)]
- Infusion Reactions [see Boxed Warning and Warnings and Precautions (5.2)]
- Stroke and Cervicocephalic Arterial Dissection [see Warnings and Precautions (5.3)]
- Malignancies [see Warnings and Precautions (5.4)]
- Immune Thrombocytopenia [see Warnings and Precautions (5.6)]
- Glomerular Nephropathies Including Anti-glomerular Basement Membrane Disease [see Warnings and Precautions (5.7)]
- Thyroid Disorders [see Warnings and Precautions (5.8)]
- Other Autoimmune Cytopenias [see Warnings and Precautions (5.9)]
- Autoimmune Hepatitis [see Warnings and Precautions (5.10)]
- Hemophagocytic Lymphohistiocytosis [see Warnings and Precautions (5.11)]
- Adult Onset Still's Disease [see Warnings and Precautions (5.12)]
- Thrombotic Thrombocytopenic Purpura (TTP) [see Warnings and Precautions (5.13)]
- Autoimmune Encephalitis (AIE) [see Warnings and Precautions (5.14)]
- Acquired Hemophilia A [see Warnings and Precautions (5.15)]
- Infections [see Warnings and Precautions (5.16)]
- Progressive Multifocal Leukoencephalopathy (PML) [see Warnings and Precautions (5.17)]
- Acute Acalculous Cholecystitis [see Warnings and Precautions (5.18)]
- Pneumonitis [see Warnings and Precautions (5.19)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled clinical trials (Study 1 and Study 2), a total of 811 patients with relapsing forms of MS received LEMTRADA. The population was 18 to 55 years of age, 65% were female, and 92% were Caucasian. A total of 811 patients received 1 course of therapy, and 789 patients received a second course of therapy at 12 months. The overall follow-up in the controlled trials was equivalent to 1622 patient years.
In MS clinical studies (controlled and open-label extension), overall, a total of 1217 patients received LEMTRADA. Approximately 60% of patients received a total of 2 treatment courses and approximately 24% of patients received a total of 3 treatment courses; others received a total of 4 or more treatment courses, although data beyond 3 treatment courses are limited. The overall follow-up was 6858 person-years. Patients had a median of 6 years of follow-up from the first LEMTRADA dose, with approximately 14% having at least 7 years of follow-up.
Most Common Adverse Reactions
In controlled clinical trials, the most common adverse reactions with LEMTRADA (in at least 10% of patients and more frequently than in interferon beta-1a) were rash, headache, pyrexia, nasopharyngitis, nausea, urinary tract infection, fatigue, insomnia, upper respiratory tract infection, herpes viral infection, urticaria, pruritus, thyroid gland disorders, fungal infection, arthralgia, pain in extremity, back pain, diarrhea, sinusitis, oropharyngeal pain, paresthesia, dizziness, abdominal pain, flushing, and vomiting.
Table 1 lists adverse reactions occurring in ≥5% of LEMTRADA-treated patients in Study 1 and 2 and at the same or at a higher rate than interferon beta-1a.
Table 1: Adverse Reactions in the Pooled 2-Year Active-Controlled Studies in Patients with Relapsing-Remitting Multiple Sclerosis|
LEMTRADA |
interferon beta-1a 44 mcg | |
|---|---|---|
|
Rash |
53 |
6 |
|
Headache |
52 |
23 |
|
Pyrexia |
29 |
9 |
|
Nasopharyngitis |
25 |
19 |
|
Nausea |
21 |
9 |
|
Urinary tract infection |
19 |
8 |
|
Fatigue |
18 |
13 |
|
Insomnia |
16 |
15 |
|
Upper respiratory tract infection |
16 |
13 |
|
Herpes viral infection |
16 |
3 |
|
Urticaria |
16 |
2 |
|
Pruritus |
14 |
2 |
|
Thyroid gland disorders |
13 |
3 |
|
Fungal infection |
13 |
4 |
|
Arthralgia |
12 |
9 |
|
Pain in extremity |
12 |
9 |
|
Back pain |
12 |
8 |
|
Diarrhea |
12 |
6 |
|
Sinusitis |
11 |
8 |
|
Oropharyngeal pain |
11 |
5 |
|
Paresthesia |
10 |
8 |
|
Dizziness |
10 |
5 |
|
Abdominal pain |
10 |
5 |
|
Flushing |
10 |
4 |
|
Vomiting |
10 |
3 |
|
Cough |
9 |
4 |
|
Chills |
9 |
3 |
|
Dysgeusia |
8 |
7 |
|
Influenza |
8 |
6 |
|
Dermatitis |
8 |
5 |
|
Dyspepsia |
8 |
4 |
|
Blood in urine |
8 |
3 |
|
Dyspnea |
8 |
1 |
|
Tachycardia |
8 |
1 |
|
Anxiety |
7 |
6 |
|
Muscular weakness |
7 |
6 |
|
Bronchitis |
7 |
4 |
|
Chest discomfort |
7 |
2 |
|
Muscle spasms |
6 |
5 |
|
Myalgia |
6 |
5 |
|
Decrease in CD4 lymphocytes |
6 |
2 |
|
Decrease in CD8 lymphocytes |
6 |
2 |
|
Asthenia |
5 |
4 |
|
Decrease in T-lymphocyte count |
5 |
3 |
|
Erythema |
5 |
2 |
|
Peripheral edema |
5 |
2 |
|
Epistaxis |
5 |
2 |
|
Neck pain |
5 |
2 |
|
Abnormal uterine bleeding |
5 |
1 |
6.2 Lymphopenia
Nearly all (99.9%) patients treated with LEMTRADA in MS clinical trials experienced lymphopenia. The lowest lymphocyte counts occurred approximately by 1 month after each course of treatment. The mean lymphocyte count at 1 month after LEMTRADA treatment was 0.25 × 109 L (range 0.02–2.30 × 109 L) and 0.32 (0.02–1.81 × 109 L) for treatment courses 1 and 2, respectively. Total lymphocyte counts increased to reach the lower limit of normal in approximately 40% of patients by 6 months after each LEMTRADA treatment course and approximately 80% of patients by 12 months after each course [see Clinical Pharmacology (12.2)].
6.3 Suicidal Behavior or Ideation
In clinical studies, 0.6% of patients in both the LEMTRADA and interferon beta-1a groups had events of attempted suicide or suicidal ideation. There were no completed suicides in either clinical study treatment group. Suicidal behavior or ideation occurred in patients with or without a history of a psychiatric or thyroid disorder. Advise patients to report immediately any symptoms of depression or suicidal ideation to the prescribing physician.
6.4 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The incidence of antibodies is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including inhibitory antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to LEMTRADA with the incidence of antibodies to other products may be misleading.
Using an enzyme-linked immunosorbent assay (ELISA) and a competitive binding assay, anti-alemtuzumab binding antibodies were detected in 62%, 67%, and 29% of LEMTRADA-treated patients, at months 1, 3, and 12 (Course 1) as well as 83%, 83%, and 75% of LEMTRADA-treated patients at months 13, 15, and 24 (Course 2). Samples that tested positive for binding antibodies were further evaluated for evidence of in vitro inhibition using a flow cytometry assay. Neutralizing antibodies were detected in 87%, 46%, and 5% of positive binding antibody patients at months 1, 3, and 12 (Course 1) as well as 94%, 88%, and 42% of positive binding antibody patients at months 13, 15, and 24 (Course 2). Anti-alemtuzumab antibodies were associated with decreased alemtuzumab concentration during Course 2, but not Course 1. Through 2 treatment courses, there was no evidence from clinical trials that the presence of binding or inhibitory anti-alemtuzumab antibodies had a significant effect on clinical outcomes, total lymphocyte count, or adverse events. High titer anti- alemtuzumab antibodies, which were observed in 13 patients, were associated with incomplete lymphocyte depletion following a third or fourth treatment course, but there was no clear effect of anti-alemtuzumab antibodies on the clinical efficacy or safety profile of LEMTRADA.
6.5 Postmarketing Experience
The following adverse reactions have been identified during post approval use of alemtuzumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Postmarketing Experience with LEMTRADA
Blood and Lymphatic System Disorders: Acquired hemophilia A [see Warnings and Precautions (5.15)], neutropenia, thrombocytopenia [see Warnings and Precautions (5.2)], thrombotic thrombocytopenic purpura [see Warnings and Precautions (5.13)]
Cerebrovascular Disorders: Stroke, including hemorrhagic and ischemic stroke and cervicocephalic arterial dissection [see Warnings and Precautions (5.3)]
Gastrointestinal System Disorders: Cholecystitis, including acalculous cholecystitis and acute acalculous cholecystitis [see Warnings and Precautions (5.18)]
Hepatobiliary Disorders: Autoimmune hepatitis [see Warnings and Precautions (5.10)], viral hepatitis [see Warnings and Precautions (5.16)]
Infections and Infestations: Opportunistic infections [see Warnings and Precautions (5.16)], Progressive multifocal leukoencephalopathy [see Warnings and Precautions (5.17)]
Immune System Disorders: Autoimmune hepatitis, vasculitis, Guillain-Barré syndrome [see Warnings and Precautions (5.1)], hemophagocytic lymphohistiocytosis [see Warnings and Precautions (5.11)], sarcoidosis
Musculoskeletal and Connective Tissue Disorders: Adult Onset Still's Disease [see Warnings and Precautions (5.12)]
Nervous System Disorders: Autoimmune encephalitis [see Warnings and Precautions (5.14)], myasthenia gravis, Lambert-Eaton myasthenic syndrome
Pulmonary System Disorders: Pulmonary alveolar hemorrhage [see Warnings and Precautions (5.2)]
Skin and Subcutaneous Tissue Disorders: Alopecia
Postmarketing Experience with CAMPATH
CAMPATH is approved for the treatment of B-cell chronic lymphocytic leukemia (B-CLL) and is generally administered at higher and more frequent doses (e.g., 30 mg) than recommended in the treatment of MS.
Cardiac Disorders: Congestive heart failure, cardiomyopathy, and decreased ejection fraction in non-MS patients previously treated with potentially cardiotoxic agents.
Most common adverse reactions (incidence ≥10% and > interferon beta-1a): rash, headache, pyrexia, nasopharyngitis, nausea, urinary tract infection, fatigue, insomnia, upper respiratory tract infection, herpes viral infection, urticaria, pruritus, thyroid gland disorders, fungal infection, arthralgia, pain in extremity, back pain, diarrhea, sinusitis, oropharyngeal pain, paresthesia, dizziness, abdominal pain, flushing, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme Corporation at 1-800-745-4447, option 2 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch**.**
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The efficacy of LEMTRADA was demonstrated in two studies (Study 1 and 2) that evaluated LEMTRADA 12 mg in patients with relapsing-remitting multiple sclerosis (RRMS). LEMTRADA was administered by intravenous infusion once daily over a 5-day course, followed one year later by intravenous infusion once daily over a 3-day course. Both studies included patients who had experienced at least 2 relapses during the 2 years prior to trial entry and at least 1 relapse during the year prior to trial entry. Neurological examinations were performed every 12 weeks and at the time of suspected relapse. Magnetic resonance imaging (MRI) evaluations were performed annually.
Study 1
Study 1 was a 2-year randomized, open-label, rater-blinded, active comparator (interferon beta-1a 44 micrograms administered subcutaneously three times a week) controlled study in patients with RRMS. Patients entering Study 1 had Expanded Disability Status Scale (EDSS) scores of 5 or less and had to have experienced at least one relapse while on interferon beta or glatiramer acetate therapy.
Patients were randomized to receive LEMTRADA (n=426) or interferon beta-1a (n=202). At baseline, the mean age was 35 years, the mean disease duration was 4.5 years, and the mean EDSS score was 2.7.
The clinical outcome measures were the annualized relapse rate (ARR) over 2 years and the time to confirmed disability progression. Confirmed disability progression was defined as at least a 1 point increase above baseline EDSS (1.5 point increase for patients with baseline EDSS of 0) sustained for 6 months. The MRI outcome measure was the change in T2 lesion volume.
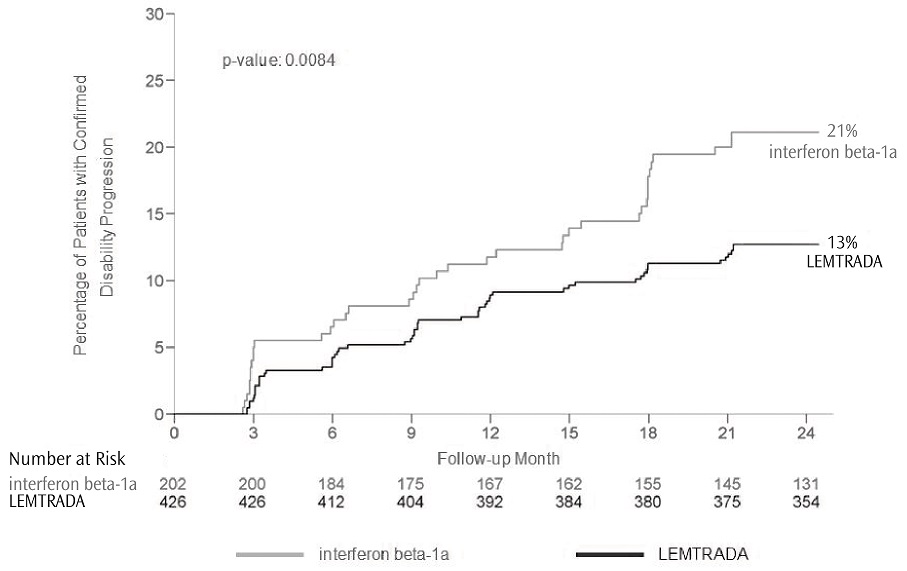
The annualized relapse rate was significantly lower in patients treated with LEMTRADA than in patients who received interferon beta-1a. Time to onset of 6-month confirmed disability progression was significantly delayed with LEMTRADA treatment compared to interferon beta-1a. There was no significant difference between the treatment groups for the change in T2 lesion volume. The results of Study 1 are shown in Table 2 and Figure 1.
Table 2: Clinical and MRI Results of Study 1|
LEMTRADA |
interferon beta-1a |
p-value | |
|---|---|---|---|
|
(N=426) |
(N=202) | ||
|
Clinical Outcomes | |||
|
Annualized relapse rate |
0.26 |
0.52 |
<0.0001 |
|
Relative reduction |
49% | ||
|
Proportion of patients with disability progression at Year 2 |
13% |
21% |
0.0084 |
|
Relative risk reduction |
42% | ||
|
Percent of patients remaining relapse-free at Year 2 |
65% |
47% |
<0.0001 |
|
MRI Outcomes | |||
|
Percent change in T2 lesion volume from baseline |
-1.3 |
-1.2 |
0.14 |
Figure 1: Time to 6-month Confirmed Disability Progression (Study 1)
Study 2
Study 2 was a 2-year randomized, open-label, rater-blinded, active comparator (interferon beta-1a 44 micrograms administered subcutaneously three times a week) controlled study in patients with RRMS. Patients entering Study 2 had EDSS scores of 3 or less and no prior treatment for multiple sclerosis.
Patients were randomized to receive LEMTRADA (n=376) or interferon beta-1a (n=187). At baseline, the mean age was 33 years, the mean disease duration was 2 years, and the mean EDSS score was 2.
The clinical outcome measures were the annualized relapse rate (ARR) over 2 years and the time to confirmed disability progression, as defined in Study 1. The MRI outcome measure was the change in T2 lesion volume.
The annualized relapse rate was significantly lower in patients treated with LEMTRADA than in patients who received interferon beta-1a. There was no significant difference between the treatment groups for the time to confirmed disability progression and for the primary MRI endpoint (change in T2 lesion volume). The results for Study 2 are shown in Table 3.
Table 3: Clinical and MRI Results of Study 2|
LEMTRADA |
interferon beta-1a |
p-value | |
|---|---|---|---|
|
(N=376) |
(N=187) | ||
|
Clinical Outcomes | |||
|
Annualized relapse rate |
0.18 |
0.39 |
<0.0001 |
|
Proportion of patients with disability progression at Year 2 |
8% |
11% |
0.22 |
|
Percent of patients remaining relapse-free at Year 2 |
78% |
59% |
<0.0001 |
|
MRI Outcomes | |||
|
Percent change in T2 lesion volume from baseline |
-9.3 |
-6.5 |
0.31 |
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Injection: 12 mg/1.2 mL (10 mg/mL) in a single-dose vial. LEMTRADA is a clear and colorless to slightly yellow solution that requires dilution prior to intravenous infusion.
Injection: 12 mg/1.2 mL (10 mg/mL) in a single-dose vial. (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of LEMTRADA in pregnant women. LEMTRADA was embryolethal in pregnant huCD52 transgenic mice when administered during organogenesis [see Animal data]. Auto-antibodies may develop after administration of LEMTRADA. Placental transfer of anti-thyroid antibodies resulting in neonatal Graves' disease has been reported.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
There is a pregnancy surveillance program for LEMTRADA. If LEMTRADA exposure occurs during pregnancy, healthcare providers and patients are encouraged to report pregnancies by calling 1-800-745-4447, option 2.
Clinical Considerations
LEMTRADA induces persistent thyroid disorders [see Warnings and Precautions (5.8)]. Untreated hypothyroidism in pregnant women increases the risk for miscarriage and may have effects on the fetus including mental retardation and dwarfism. In mothers with Graves' disease, maternal thyroid stimulating hormone receptor antibodies can be transferred to a developing fetus and can cause neonatal Graves' disease. In a patient who developed Graves' disease after treatment with alemtuzumab, placental transfer of anti-thyrotropin receptor antibodies resulted in neonatal Graves' disease with thyroid storm in her infant who was born 1 year after alemtuzumab dosing [see Warnings and Precautions (5.1)].
Data
Animal data
When LEMTRADA was administered to pregnant huCD52 transgenic mice during organogenesis (gestation days [GD] 6–10 or GD 11–15) at doses of 3 or 10 mg/kg IV, no teratogenic effects were observed. However, there was an increase in embryolethality (increased postimplantation loss and the number of dams with all fetuses dead or resorbed) in pregnant animals dosed during GD 11–15. In a separate study in pregnant huCD52 transgenic mice, administration of LEMTRADA during organogenesis (GD 6–10 or GD 11–15) at doses of 3 or 10 mg/kg IV, decreases in B- and T-lymphocyte populations were observed in the offspring at both doses tested.
In pregnant huCD52 transgenic mice administered LEMTRADA at doses of 3 or 10 mg/kg/day IV throughout gestation and lactation, there was an increase in pup deaths during the lactation period at 10 mg/kg. Decreases in T- and B-lymphocyte populations and in antibody response were observed in offspring at both doses tested.
8.2 Lactation
Risk Summary
There are no data on the presence of alemtuzumab in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. Alemtuzumab was detected in the milk of lactating huCD52 transgenic mice administered LEMTRADA [see Animal data].
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for LEMTRADA and any potential adverse effects on the breastfed child from LEMTRADA or from the underlying maternal conditions.
Data
Animal data
Alemtuzumab was detected in the milk of lactating huCD52 transgenic mice following intravenous administration of LEMTRADA at a dose of 10 mg/kg on postpartum days 8–12. Serum levels of alemtuzumab were similar in lactating mice and offspring on postpartum Day 13 and were associated with evidence of pharmacological activity (decrease in lymphocyte counts) in the offspring.
8.3 Females and Males of Reproductive Potential
Contraception
Before initiation of LEMTRADA treatment, women of childbearing potential should be counselled on the potential for a serious risk to the fetus. To avoid in utero exposure to LEMTRADA, women of childbearing potential should use effective contraceptive measures when receiving a course of treatment with LEMTRADA and for 4 months following that course of treatment [see Use in Specific Populations (8.1)].
Infertility
In huCD52 transgenic mice, administration of LEMTRADA prior to and during the mating period resulted in adverse effects on sperm parameters in males and reduced number of corpora lutea and implantations in females [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients less than 17 years of age have not been established. Use of LEMTRADA is not recommended in pediatric patients due to the risks of autoimmunity, infusion reactions, and stroke, and because it may increase the risk of malignancies (thyroid, melanoma, lymphoproliferative disorders, and lymphoma) [see Warnings and Precautions (5.1, 5.2, 5.3, 5.4)].
8.5 Geriatric Use
Clinical studies of LEMTRADA did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients.
Pregnancy: May cause fetal harm. (8.1)
Women of childbearing potential should use effective contraception during and for 4 months after a course of treatment with LEMTRADA. (8.3)
OVERDOSAGE SECTION
10 OVERDOSAGE
Two MS patients experienced serious reactions (headache, rash, and either hypotension or sinus tachycardia) after a single accidental infusion up to 60 mg of LEMTRADA. Doses of LEMTRADA greater than those recommended may increase the intensity and/or duration of infusion reactions or its immune effects. There is no known antidote for alemtuzumab overdosage.
DESCRIPTION SECTION
11 DESCRIPTION
Alemtuzumab is a recombinant humanized IgG1 kappa monoclonal antibody directed against the cell surface glycoprotein, CD52. Alemtuzumab has an approximate molecular weight of 150 kD. Alemtuzumab is produced in mammalian cell (Chinese hamster ovary) suspension culture in a nutrient medium containing neomycin. Neomycin is not detectable in the final product.
LEMTRADA (alemtuzumab) injection is a sterile, clear and colorless to slightly yellow, solution (pH 7.2 ± 0.2) for intravenous infusion.
Each 1 mL of solution contains 10 mg alemtuzumab, dibasic sodium phosphate (1.15 mg), disodium edetate dihydrate (0.0187 mg), polysorbate 80 (0.1 mg), potassium chloride (0.2 mg), potassium dihydrogen phosphate (0.2 mg), sodium chloride (8 mg), and Water for Injection, USP.
SPL UNCLASSIFIED SECTION
Manufactured and distributed by:
Genzyme Corporation
Cambridge, MA 02141
A SANOFI COMPANY
US License Number: 1596
LEMTRADA and CAMPATH are registered trademarks of Genzyme Corporation. ©2024 Genzyme Corporation.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Autoimmunity
- Advise patients to contact their healthcare provider promptly if they experience any symptoms of potential autoimmune disease. Give examples of important symptoms such as bleeding, easy bruising, petechiae, purpura, hematuria, edema, jaundice, or hemoptysis [see Warnings and Precautions (5.1)].
- Advise patients of the importance of monthly blood and urine tests for 48 months following the last course of LEMTRADA to monitor for signs of autoimmunity because early detection and prompt treatment can help prevent serious and potentially fatal outcomes associated with these events. Advise patients that monitoring may need to continue past 48 months if they have signs or symptoms of autoimmunity.
- Advise patients that LEMTRADA may cause hyperthyroid or hypothyroid disorders.
- Advise patients to contact their healthcare provider if they experience symptoms reflective of a potential thyroid disorder such as unexplained weight loss or gain, fast heartbeat or palpitations, nervousness, worsening tiredness, eye swelling, constipation, or feeling cold.
- Advise women of childbearing potential of the risks of pregnancy with concomitant thyroid disease. Advise women of childbearing potential to discuss pregnancy planning with their doctor.
- Cases of autoimmune hepatitis have been reported in patients treated with LEMTRADA. Advise patients to contact their healthcare provider right away if they develop signs or symptoms suggestive of hepatic dysfunction such as unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice and/or dark urine, or bleeding or bruising more easily than normal.
- Advise patients to contact their healthcare provider if they experience symptoms of acquired hemophilia A such as spontaneous bruising, nose bleeds, painful or swollen joints, other types of bleeding, or bleeding from a cut that may take longer than usual to stop.
- Advise patients that cases of autoimmune encephalitis can occur after receiving LEMTRADA. This condition may include symptoms such as behavior and psychiatric changes, movement disorders, short-term memory loss or seizures, as well as other symptoms that may resemble an MS relapse.
Infusion Reactions
- Advise patients that infusion reactions can occur at the time of infusion or after they leave the infusion center [see Warnings and Precautions (5.2)].
- Instruct the patient to remain at the infusion center for at least 2 hours after each LEMTRADA infusion, or longer at the discretion of the healthcare provider. Advise patients that symptoms of infusion reactions may occur after they leave the infusion center and to report these symptoms to their healthcare provider.
- Advise patients that their healthcare provider will monitor vital signs, including blood pressure, before and during the infusion and to contact their healthcare provider promptly if they experience infusion reactions, which include swelling in the mouth or throat, difficulty breathing, weakness, abnormal heart rate (fast, slow, or irregular), chest pain, rash, facial drooping, sudden severe headache, weakness on one side of the body, difficulty with speech, or neck pain.
- Instruct patients that there have also been reports of rare but serious infusion reactions, including bleeding in the lung, chest tightness/pain or discomfort, heart attack, and stroke or tears in blood vessels supplying the brain, which should be reported to your healthcare provider.
- Advise patients that reactions may occur following any of the doses during the treatment course. In the majority of cases, reactions occurred within 1–3 days of the infusion.
Stroke and Cervicocephalic Arterial Dissection
- Educate patients on the symptoms and instruct patients to seek immediate medical attention if symptoms of stroke or cervicocephalic arterial dissection occur (e.g., neck pain, weakness on one side, facial droop, difficulty with speech, sudden severe headache) [see Warnings and Precautions (5.3)].
Malignancies
- Advise patients that LEMTRADA may increase their risk of malignancies including thyroid cancer and melanoma [see Warnings and Precautions (5.4)].
- Advise patients to report symptoms of thyroid cancer, including a new lump or swelling in the neck, pain in the front of the neck, hoarseness or other voice changes that do not go away, trouble swallowing or breathing, or a constant cough not due to a cold.
- Advise patients that they should have baseline and yearly skin examinations.
LEMTRADA REMS Program
- LEMTRADA is available only through a restricted program called the LEMTRADA REMS Program [see Warnings and Precautions (5.5)]. Inform the patient of the following notable requirements:
- Patients and providers must be enrolled in the program.
- Patients must comply with the ongoing monitoring requirements.
- Patients must report any side effects or symptoms to their doctor.
- LEMTRADA is available only at certified infusion centers participating in the program. Therefore, provide patients with information on the LEMTRADA REMS Program in order to locate an infusion center.
- Advise patients to read the LEMTRADA REMS material for patients, LEMTRADA Treatment and Infusion Reactions Patient Guide.
- Instruct patients to carry the LEMTRADA REMS Patient Safety Information Card with them in case of an emergency.
Hemophagocytic Lymphohistiocytosis
- Inform patients that treatment with LEMTRADA may increase the risk of a type of excessive immune activation (hemophagocytic lymphohistiocytosis), which can be fatal, particularly if not diagnosed and treated early.
- Advise patients to contact their healthcare provider immediately if they experience symptoms such as fever, swollen glands, skin rash, or new neurologic symptoms such as mental status changes, ataxia, or seizures.
- In cases reported with LEMTRADA, symptoms occurred within approximately thirteen months to thirty-three months following the initiation of treatment.
Adult Onset Still's Disease (AOSD)
- Inform patients that AOSD is a rare condition that has the potential to cause multi-organ inflammation with several symptoms such as fever >39°C or 102.2°F lasting more than 1 week, pain, stiffness with or without swelling in multiple joints, and/or a skin rash [see Warnings and Precautions (5.12)].
- Instruct patients if they experience a combination of these symptoms to contact their healthcare provider immediately.
Thrombotic Thrombocytopenic Purpura
- Inform patients that there have been reports of TTP in patients treated with LEMTRADA and that this is a potentially life-threatening condition [see Warnings and Precautions (5.13)].
- Instruct patients to get prompt medical attention if they experience symptoms of TTP such as fever, fatigue, pallor, purpura, jaundice, tachycardia, dyspnea, hematuria, dark-colored urine, decreased urine volume, abdominal pain, nausea, vomiting, or new neurological symptoms such as confusion, altered mental status, vision or speech changes, or seizures.
Infections
- Advise patients to contact their healthcare provider if they develop symptoms of serious infection such as fatigue, fever, or swollen glands [see Warnings and Precautions (5.16)].
- Advise patients to complete any necessary immunizations at least 6 weeks prior to treatment with LEMTRADA [see Dosage and Administration (2.1)]. Advise patients that they should talk to their healthcare provider before taking any vaccine after recent treatment with LEMTRADA [see Warnings and Precautions (5.16)].
- Advise patients to avoid or adequately heat foods that are potential sources of Listeria monocytogenes prior to receiving LEMTRADA and if they have had a recent course of LEMTRADA. The duration of increased risk for Listeria infection after LEMTRADA administration is not known. Inform patients that Listeria infection can lead to significant complications or death [see Warnings and Precautions (5.16)].
- Advise patients to take their prescribed medication for herpes prophylaxis as directed by their healthcare provider [see Warnings and Precautions (5.16)].
- Advise patients that yearly HPV screening is recommended [see Warnings and Precautions (5.16)].
Progressive Multifocal Leukoencephalopathy
- Inform patients that progressive multifocal leukoencephalopathy (PML) has occurred in a patient who received LEMTRADA. Inform the patient that PML is characterized by a progression of deficits and usually leads to death or severe disability over weeks or months. Instruct the patient of the importance of contacting their doctor if they develop any symptoms suggestive of PML. Inform the patient that typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes [see Warnings and Precautions (5.17)].
Acute Acalculous Cholecystitis
- Advise patients to report symptoms of acute acalculous cholecystitis. These include abdominal pain, abdominal tenderness, fever, nausea, and vomiting [see Warnings and Precautions (5.18)].
Pneumonitis
- Advise patients that pneumonitis has been reported in patients treated with LEMTRADA [see Warnings and Precautions (5.19)]. Advise patients to report symptoms of lung disease such as shortness of breath, cough, wheezing, chest pain or tightness, and hemoptysis.
Concomitant Use of CAMPATH
- Advise patients that alemtuzumab is the same drug as CAMPATH for use in B-CLL. Patients should inform their healthcare provider if they have taken CAMPATH [see Warnings and Precautions (5.20)].
Fetal Risk
- Inform patients that LEMTRADA may cause fetal harm. Discuss with women of childbearing age whether they are pregnant, might be pregnant, or are trying to become pregnant. Advise women of childbearing age of the need for effective contraception during LEMTRADA treatment and for 4 months after a treatment course of LEMTRADA. Advise the patient that if she should nevertheless become pregnant, she should immediately inform her physician.
- Advise patients exposed to LEMTRADA during pregnancy that there is a pregnancy safety surveillance program that monitors pregnancy outcomes [see Use in Specific Populations (8.1)]. If exposure occurs during pregnancy, healthcare providers and patients are encouraged to report pregnancies by calling 1-800-745-4447, option 2.
SPL MEDGUIDE SECTION
|
This Medication Guide has been approved by the U.S. Food and Drug Administration |
Revised: February 2024 | |
|
MEDICATION GUIDE | ||
|
Read this Medication Guide before you start receiving LEMTRADA and before you begin each treatment course. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. | ||
|
What is the most important information I should know about LEMTRADA? LEMTRADA can cause serious side effects, including: *immune thrombocytopenic purpura (ITP). LEMTRADA may cause the number of platelets in your blood to be reduced (ITP). ITP can cause severe bleeding that may cause life-threatening problems. Call your healthcare provider right away if you have any of the following symptoms: | ||
|
| |
|
*kidney problems. LEMTRADA may cause a serious kidney problem called anti-glomerular basement membrane disease. If this happens and you do not get treated, anti-glomerular basement membrane disease can lead to severe kidney damage, kidney failure that needs dialysis, a kidney transplant, or death. Call your healthcare provider right away if you have any of the following symptoms: | ||
|
| |
|
Side effects may happen while you receive LEMTRADA and for 4 years after you stop receiving LEMTRADA. Your healthcare provider will order blood and urine tests before you receive, while you are receiving, and every month for 4 years after you receive your last LEMTRADA infusion. You may need to continue these blood and urine tests after 4 years if you have any autoimmune signs or symptoms. The blood and urine tests will help your healthcare provider watch for signs and symptoms of serious autoimmune problems. It is important to have your blood and urine tested, even if you are feeling well and do not have any symptoms from LEMTRADA and your multiple sclerosis. This may help your healthcare provider find any problems early. You will receive your infusion at a healthcare facility with equipment and
staff trained to manage infusion reactions. You will be watched while you
receive and for at least2 hours after you receive LEMTRADA.It is
important that you stay at the infusion center for at least2 hours
after your infusion is finished or longer if your healthcare provider decides
you need to stay longer. If a serious infusion reaction happens while you are
receiving LEMTRADA, your infusion may be stopped. | ||
|
| |
|
To lower your chances of getting a serious infusion reaction, your healthcare provider will give you a medicine called corticosteroids before your first 3 infusions of a treatment course. You may also be given other medicines before or after the infusion to try to reduce your chances of these reactions or to treat them after they happen. | ||
|
| |
| ||
|
| |
|
You should have your skin checked before you start receiving LEMTRADA and each year while you are receiving treatment to monitor symptoms of skin cancer. Because of your risk of autoimmunity, infusion reactions, and the risk of some kinds of cancers, LEMTRADA is only available through a restricted program called the LEMTRADA Risk Evaluation and Mitigation Strategy (REMS) Program. Call 1-855-676-6326 to enroll in the LEMTRADA REMS Program.
| ||
|
What is LEMTRADA? LEMTRADA is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Since treatment with LEMTRADA can increase your risk of getting certain conditions and diseases, LEMTRADA is generally prescribed for people who have tried 2 or more MS medicines that have not worked well enough. LEMTRADA is not recommended for use in patients with clinically isolated syndrome (CIS). It is not known if LEMTRADA is safe and effective for use in children under 17 years of age. | ||
|
Who should not receive LEMTRADA? Do not receive LEMTRADA if you:
| ||
|
What should I tell my healthcare provider before receiving LEMTRADA? Before receiving LEMTRADA, tell your healthcare provider if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. LEMTRADA and other medicines may affect each other causing side effects. Especially tell your healthcare provider if you take medicines that increase your chance of getting infections, including medicines used to treat cancer or to control your immune system. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | ||
|
How will I receive LEMTRADA?
| ||
|
What are the possible side effects of LEMTRADA? LEMTRADA may cause serious side effects including:
| ||
|
– – – |
– – | |
| ||
|
– – |
– – | |
|
*low blood counts (cytopenias). LEMTRADA may cause a decrease in some types of blood cells. Some people with these low blood counts have increased infections. Symptoms of cytopenias may include: | ||
|
| |
|
Your healthcare provider will do blood tests to check for cytopenias. Call your healthcare provider right away if you have symptoms listed above. *inflammation of the liver. Call your healthcare provider right away if you have symptoms such as unexplained nausea, stomach pain, tiredness, loss of appetite, yellowing of skin or whites of eyes, or bleeding or bruising more easily than normal. *hemophagocytic lymphohistiocytosis (HLH). LEMTRADA may increase the risk of a type of overactivity of the immune system (hemophagocytic lymphohistiocytosis) that can be fatal, especially if not diagnosed and treated early. Call your healthcare provider right away if you have symptoms such as fever, swollen glands, skin rash, or new nervous system problems, such as seizures, changes in your thinking or level of alertness, or new or worsening unsteadiness or trouble walking. These symptoms have happened in people taking LEMTRADA about 13 months to 33 months after they started taking LEMTRADA. *adult onset still's disease (AOSD). Adult onset still's disease (AOSD) is a rare condition that can cause a high fever lasting more than 1 week, pain, stiffness with or without swelling in multiple joints, and/or a skin rash. If you experience a combination of these symptoms, contact your healthcare provider immediately. *thrombotic thrombocytopenic purpura (TTP). Thrombotic thrombocytopenic purpura (TTP) can occur with LEMTRADA. TTP is a blood clotting problem where blood clots can form in blood vessels anywhere in the body. TTP needs to be treated in a hospital right away, because it can cause death. Get medical help right away if you have any of these symptoms: | ||
|
| |
|
*autoimmune encephalitis (AIE). Autoimmune encephalitis (AIE), a brain disorder, can occur after receiving LEMTRADA and may include symptoms that may seem like an MS relapse. Call your healthcare provider right away if you have any of the following symptoms: | ||
|
| |
|
*bleeding disorder (acquired hemophilia A). LEMTRADA may cause a bleeding disorder called acquired hemophilia A. Call your healthcare provider right away if you have any of the following symptoms: | ||
|
| |
|
*serious infections. LEMTRADA may cause you to have serious infections while you receive and after receiving a treatment course. Serious infections may include: *listeria. People who receive LEMTRADA have an increased chance of getting an infection caused by the bacteria, listeria, which can lead to significant complications or death. Avoid foods that may be a source for listeria (for example, deli meat, unpasteurized milk and cheese products, soft cheeses, or undercooked meat, seafood or poultry) or make sure that the food you eat which may contain listeria is heated well if you receive treatment with LEMTRADA. *herpes viral infections. Some people taking LEMTRADA have an increased chance of getting herpes viral infections. Your healthcare provider will prescribe medicines to reduce your chances of getting these infections. Take these medicines exactly as your healthcare provider tells you to. *human papilloma virus (HPV). Females have an increased chance of getting a cervical HPV infection. If you are a female, you should have an HPV screening each year. *tuberculosis. Your healthcare provider should check you for tuberculosis before you receive LEMTRADA. *fungal infections. Call your healthcare provider right away if you have symptoms of a serious infection, such as fever or swollen glands. You may need to go to the hospital for treatment if you get a serious infection. It is important to tell the healthcare providers that you have received LEMTRADA. Talk to your healthcare provider before you get vaccinations after receiving LEMTRADA. Certain vaccinations may increase your chances of getting infections. | ||
|
*Progressive multifocal leukoencephalopathy (PML). A rare brain infection that usually leads to death or severe disability has been reported with LEMTRADA. Symptoms of PML get worse over days to weeks. It is important that you call your doctor right away if you have any new or worsening medical problems that have lasted several days, including problems with: | ||
|
| |
|
*Inflammation of the gallbladder without gallstones (acalculous cholecystitis). LEMTRADA may increase your chance of getting inflammation of the gallbladder without gallstones, a serious medical condition that can be life-threatening. Call your healthcare provider right away if you have any of the following symptoms of acalculous cholecystitis, which may include: | ||
|
| |
|
*swelling of lung tissue (pneumonitis). Some people have had swelling of the lung tissue while receiving LEMTRADA. Call your healthcare provider right away if you have the following symptoms: | ||
|
| |
|
The most common side effects of LEMTRADA include: | ||
|
| |
|
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of LEMTRADA. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088. | ||
|
General information about the safe and effective use of LEMTRADA. This Medication Guide summarizes the most important information about LEMTRADA. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about LEMTRADA that is written for health professionals. For more information, go to www.LemtradaREMS.com or call Genzyme at 1-855-676-6326. | ||
|
What are the ingredients in LEMTRADA? Active ingredient: alemtuzumab Inactive ingredients: dibasic sodium phosphate, disodium edetate dihydrate, polysorbate 80, potassium chloride, potassium dihydrogen phosphate, sodium chloride, and Water for Injection, USP. | ||
|
Manufactured and distributed by: LEMTRADA and CAMPATH are registered trademarks of Genzyme Corporation. |
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Testing and Procedures Prior to Treatment
Baseline laboratory tests are required prior to treatment with LEMTRADA [see Dosage and Administration (2.6)]. In addition, prior to starting treatment with LEMTRADA [see Warnings and Precautions (5.15)]:
- complete any necessary immunizations at least 6 weeks prior to treatment.
- determine whether patients have a history of varicella or have been vaccinated for varicella zoster virus (VZV). If not, test the patient for antibodies to VZV and consider vaccination for those who are antibody-negative. Postpone treatment with LEMTRADA until 6 weeks after VZV vaccination.
- perform tuberculosis screening according to local guidelines.
- instruct patients to avoid potential sources of Listeria monocytogenes.
2.2 Recommended Premedication and Concomitant Medication
Corticosteroids
Premedicate patients with high dose corticosteroids (1,000 mg methylprednisolone or equivalent) immediately prior to LEMTRADA infusion and for the first 3 days of each treatment course [see Warnings and Precautions (5.2)].
Herpes Prophylaxis
Administer antiviral prophylaxis for herpetic viral infections starting on the first day of each treatment course and continue for a minimum of two months following treatment with LEMTRADA or until the CD4+ lymphocyte count is at least 200 cells per microliter, whichever occurs later [see Warnings and Precautions (5.15)].
2.3 Recommended Dosage
- The recommended dosage of LEMTRADA is 12 mg/day administered by intravenous infusion for 2 treatment courses: First Treatment Course: 12 mg/day on 5 consecutive days (60 mg total dose).
- Second Treatment Course: 12 mg/day on 3 consecutive days (36 mg total dose) administered 12 months after the first treatment course.
Following the second treatment course, subsequent treatment courses of 12 mg per day on 3 consecutive days (36 mg total dose) may be administered, as needed, at least 12 months after the last dose of any prior treatment courses.
2.4 Preparation Instructions
Follow the steps below to prepare the diluted solution of LEMTRADA for intravenous infusion:
- Inspect LEMTRADA visually for particulate matter and discoloration prior to administration. Do not use if particulate matter is present or the solution is discolored. Do not freeze or shake vials prior to use.
- Withdraw 1.2 mL of LEMTRADA from the vial into a syringe using aseptic technique and inject into a 100 mL bag of sterile 0.9% Sodium Chloride, USP or 5% Dextrose in Water, USP.
- Gently invert the bag to mix the solution. Ensure the sterility of the prepared solution because it contains no antimicrobial preservatives. Each vial is for single use only.
Prior to administration, protect diluted LEMTRADA solution from light and store for as long as 8 hours either at room temperature 15°C to 25°C (59°F to 77°F) or keep refrigerated at conditions 2°C to 8°C (36°F to 46°F).
2.5 Infusion Instructions
Infuse LEMTRADA over 4 hours starting within 8 hours after dilution. Extend the duration of the infusion if clinically indicated.
Administer LEMTRADA in a setting in which equipment and personnel to appropriately manage anaphylaxis, serious infusion reactions, myocardial ischemia, myocardial infarction, and cerebrovascular and respiratory adverse reactions are available [see Warnings and Precautions (5.2)].
Do not add or simultaneously infuse other drug substances through the same intravenous line. Do not administer as an intravenous push or bolus.
Obtain a baseline ECG. Monitor vital signs before the infusion and periodically during the infusion. Provide appropriate symptomatic treatment for infusion reactions as needed. Consider immediate discontinuation of the intravenous infusion if severe infusion reactions occur.
Observe patients for infusion reactions during and for at least 2 hours after each LEMTRADA infusion. Consider longer periods of observation if clinically indicated. Inform patients that they should report symptoms that occur during and after each infusion because they may indicate a need for prompt medical intervention [see Warnings and Precautions (5.2)].
2.6 Laboratory Testing and Monitoring to Assess Safety
Measure the urine protein to creatinine ratio prior to initiation of treatment. Conduct the following laboratory tests at baseline and at periodic intervals until 48 months after the last treatment course of LEMTRADA in order to monitor for early signs of potentially serious adverse effects:
- Complete blood count (CBC) with differential (prior to treatment initiation and at monthly intervals thereafter)
- Serum creatinine levels (prior to treatment initiation and at monthly intervals thereafter)
- Urinalysis with urine cell counts (prior to treatment initiation and at monthly intervals thereafter)
- A test of thyroid function, such as thyroid stimulating hormone (TSH) level (prior to treatment initiation and every 3 months thereafter)
- Serum transaminases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) and total bilirubin levels (prior to treatment initiation and periodically thereafter)
Conduct baseline and yearly skin exams to monitor for melanoma [see Warnings and Precautions (5.4)].
-
Baseline laboratory tests are required prior to treatment. (2.1)
-
Administer LEMTRADA by intravenous infusion over 4 hours for 2 or more treatment courses:
Initial treatment of 2 courses:- First course: 12 mg/day on 5 consecutive days. (2.3)
- Second course: 12 mg/day on 3 consecutive days 12 months after first treatment course. (2.3) Subsequent treatment courses of 12 mg per day on 3 consecutive days (36 mg total dose) may be administered, as needed, at least 12 months after the last dose of any prior treatment course. (2.3)
-
Premedicate with corticosteroids prior to LEMTRADA infusion for the first 3 days of each treatment course. (2.2)
-
Administer antiviral agents for herpetic prophylaxis starting on the first day of LEMTRADA dosing and continuing for a minimum of two months after completion of LEMTRADA dosing or until CD4+ lymphocyte count is more than 200 cells per microliter, whichever occurs later. (2.2)
-
Must be diluted prior to administration. (2.4)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism by which alemtuzumab exerts its therapeutic effects in multiple sclerosis is unknown but is presumed to involve binding to CD52, a cell surface antigen present on T and B lymphocytes, and on natural killer cells, monocytes, and macrophages. Following cell surface binding to T and B lymphocytes, alemtuzumab results in antibody-dependent cellular cytolysis and complement-mediated lysis.
12.2 Pharmacodynamics
Effects of LEMTRADA on the Lymphocyte Population
LEMTRADA depletes circulating T and B lymphocytes after each treatment course. In clinical trials, the lowest cell counts occurred 1 month after a course of treatment at the time of the first post-treatment blood count. Lymphocyte counts then increased over time: B cell counts usually recovered within 6 months; T cell counts increased more slowly and usually remained below baseline 12 months after treatment. Approximately 60% of patients had total lymphocyte counts below the lower limit of normal 6 months after each treatment course and 20% had counts below the lower limit of normal after 12 months.
Reconstitution of the lymphocyte population varies for the different lymphocyte subtypes. At Month 1 in clinical trials, the mean CD4+ lymphocyte count was 40 cells per microliter, and, at Month 12, 270 cells per microliter. At 30 months, approximately half of patients had CD4+ lymphocyte counts that remained below the lower limit of normal.
Cardiac Electrophysiology
In a study of 53 MS patients, alemtuzumab 12 mg per day for 5 days caused no changes in the QTc interval greater than 20 ms. An average 22 to 26 beats-per- minute increase in heart rate was observed for at least 2 hours after the first but not subsequent infusions.
12.3 Pharmacokinetics
The pharmacokinetics of LEMTRADA were evaluated in a total of 148 patients with relapsing forms of MS who received 12 mg/day on 5 consecutive days, followed by 12 mg/day on 3 consecutive days 12 months following the first treatment course.
Absorption
Serum concentrations increased with each consecutive dose within a treatment course, with the highest observed concentrations occurring following the last infusion of a treatment course. The mean maximum concentration was 3014 ng/mL on Day 5 of the first treatment course, and 2276 ng/mL on Day 3 of the second treatment course.
Distribution
LEMTRADA is largely confined to the blood and interstitial space with a central volume of distribution of 14.1 L.
Elimination
The elimination half-life was approximately 2 weeks and was comparable between courses. The serum concentrations were generally undetectable (<60 ng/mL) within approximately 30 days following each treatment course.
Specific Populations
Age, race, or gender had no effect on the pharmacokinetics of LEMTRADA.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to assess the carcinogenic or genotoxic potential of LEMTRADA have not been conducted.
When LEMTRADA (3 or 10 mg/kg IV) was administered to huCD52 transgenic male mice on 5 consecutive days prior to cohabitation with untreated wild-type females, no effect on fertility or reproductive performance was observed. However, adverse effects on sperm parameters (including abnormal morphology [detached/no head] and reduced total count and motility) were observed at both doses tested.
When LEMTRADA (3 or 10 mg/kg IV) was administered to huCD52 transgenic female mice for 5 consecutive days prior to cohabitation with untreated wild-type males, there was a decrease in the average number of corpora lutea and implantation sites and an increase in postimplantation loss, resulting in fewer viable embryos at the higher dose tested.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
LEMTRADA (alemtuzumab) injection is a sterile, clear and colorless to slightly yellow solution for intravenous infusion, containing no antimicrobial preservatives.
Each LEMTRADA carton (NDC: 58468-0200-1) contains one single-dose vial that delivers 12 mg/1.2 mL (10 mg/mL). The vial stopper is not made with natural rubber latex.
16.2 Storage and Handling
Store LEMTRADA vials at 2°C to 8°C (36°F to 46°F). Do not freeze or shake. Store in original carton to protect from light.
