Nystatin
Nystatin Tablets USP Rx only
8fd35f97-5405-2160-ca0f-35034ecd5a92
HUMAN PRESCRIPTION DRUG LABEL
Sep 18, 2025
AvKARE
DUNS: 796560394
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nystatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Nystatin is well tolerated even with prolonged therapy. Oral irritation and sensitization have been reported (seePRECAUTIONS, General).
Gastrointestinal
Diarrhea (including one case of bloody diarrhea), nausea, vomiting, gastrointestinal upset/disturbances.
Dermatologic
Rash, including urticaria has been reported rarely. Stevens-Johnson syndrome has been reported very rarely.
Other
Tachycardia, bronchospasm, facial swelling, and nonspecific myalgia have also been rarely reported.
To report SUSPECTED ADVERSE REACTIONS contact AvKARE at 1-855-361-3993; email drugsafety@avkare.com; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
DESCRIPTION
Nystatin, USP is an antimycotic polyene antibiotic obtained from Streptomyces noursei. Its structural formula:
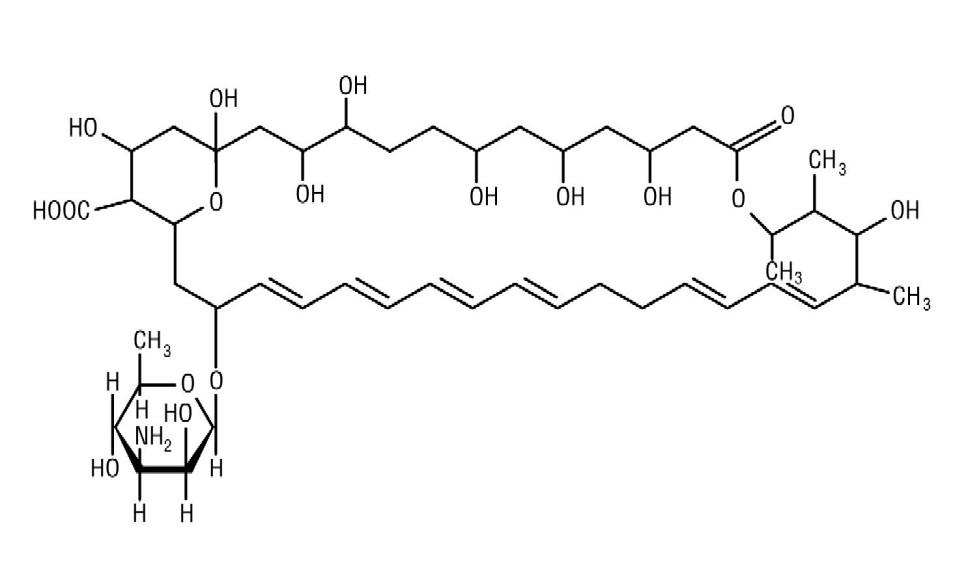
C 47H 75NO 17 M.W. 926.13
Nystatin tablets USP contain the inactive ingredients: Corn Starch, Povidone, Compressible Sugar, Microcrystalline Cellulose, Sodium Starch Glycolate, Talc, Magnesium Stearate, Purified Water, and Coloring.
HOW SUPPLIED SECTION
HOW SUPPLIED
Nystatin Tablets USP, 500,000 Units are round, convex, brown, film-coated tablet debossed with 93 on one side and 983 on the reverse and are packaged in bottles of 90 tablets (NDC 42291-651-90).
Store at 20° and 25°C (68° and 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Keep tightly closed.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured For:
AvKARE
Pulaski, TN 38478
Mfg. Rev. 11/22
AV Rev. 09/25(M)
