Chlorzoxazone
Revised: March 2015 195609-1
6c418b8f-4a1c-4611-80d5-3a3a7dbca9c8
HUMAN PRESCRIPTION DRUG LABEL
Mar 8, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Chlorzoxazone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Chlorzoxazone USP is a centrally acting skeletal muscle relaxant, available as tablets of 500 mg for oral administration. Its chemical name is 5-Chloro-2-benzoxazolinone, and its structural formula is:
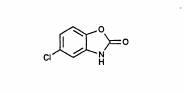
Chlorzoxazone Structural Formula
C7H4CINO2 MW 169.57
Chlorzoxazone USP is a white or practically white, practically odorless, crystalline powder. Chlorzoxazone is slightly soluble in water; sparingly soluble in alcohol, in isopropyl alcohol, and in methanol; soluble in solutions of alkali hydroxides and ammonia.
Chlorzoxazone tablets contain the inactive ingredients Docusate Sodium, Lactose (hydrous), Magnesium Stearate, Microcrystalline Cellulose, Pregelatinized Starch, Sodium Benzoate, and Sodium Starch Glycolate.
