hydroxyzine pamoate
Hydroxyzine Pamoate Capsules, USP
db0d9f72-f5b5-48f7-e053-2995a90af2b6
HUMAN PRESCRIPTION DRUG LABEL
Jan 6, 2023
Denton Pharma, Inc. DBA Northwind Pharmaceuticals
DUNS: 080355546
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
hydroxyzine pamoate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Hydroxyzine pamoate is a light yellow, practically odorless powder practically insoluble in water and methanol and freely soluble in dimethylformamide. It is chemically designated as (±)-2-[2-[4-( p-Chloro-α- phenylbenzyl)-1-piperazinyl]ethoxy]ethanol 4,4’-methylenebis[3-hydroxy-2-naphthoate] (1:1) [10246-75-0] and can be structurally represented as follows:
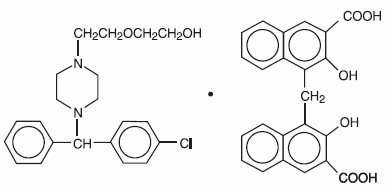
C 21H 27CIN 2O 2•C 23H 16O 6
M.W. 763.27
Each capsule, for oral administration, contains hydroxyzine pamoate equivalent to hydroxyzine hydrochloride 25 mg or 50 mg.
In addition, each capsule contains the following inactive ingredients: colloidal silicon dioxide, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, sodium starch glycolate (potato), and sodium lauryl sulfate.
The capsule shell contains the following ingredients: D&C Yellow #10, FD&C Green #3, FD&C Yellow #6, gelatin, and titanium dioxide.
The edible imprinting ink contains the following ingredients: black iron oxide, D&C Yellow #10, FD&C Blue #1, FD&C Blue #2, FD&C Red #40, propylene glycol, and shellac glaze.
